Detergents are amphipathic compounds with a nonpolar, hydrophobic tail and a polar, hydrophilic head group. Due to these structural features detergents tend to aggregate into structures called micelles at high enough concentration; arranging themselves with their hydrophobic tails pointed inwards and their hydrophilic heads pointed outwards. Detergents come in three types: ionic (cationic and anionic) and non-ionic. Non-ionic detergents aren’t generally used for gel electrophoresis due to their limited ability to break non-covalent interactions between protein residues and inability to impart a uniform charge onto the protein. Ionic detergents (typically anionic SDS) are used for gel electrophoresis as they are highly useful for protein solubilization, linearization and for establishing a uniform charge in preparation for gel electrophoresis.
Topics: Protein Purification, Western Blotting, Protein Estimation, Detergents, Sample Clean Up, Protein Concentration, Protein Fractionation, Protein Labeling, Protein Extraction, Protein Detection
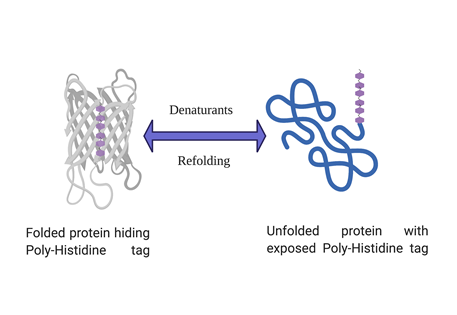
His-tagged protein expressing but not binding your Ni-NTA column?
Poly-histidine tagged proteins account for more than 90% of routine recombinant protein expression and purification. Histidine tag has many advantages over other affinity tags such as small size, high affinity for binding to IMAC resins (Immobilized metal affinity chromatography) and allows one step purification of recombinant protein. But sometimes a histidine tagged protein doesn’t bind to the affinity column and evades it’s capture and subsequent purification. Although such instances are not very common but can be very frustrating whenever they happen. However, there are ways to troubleshoot if such a problem arises and the solutions are discussed below in a step by step manner.
Topics: Protein Purification, Western Blotting, Protein Electrophoresis, Sample Clean Up, Protein Concentration, Protein Fractionation, Protein Labeling, Protein Extraction, Protein Detection
Guess what??? Immobilized Papain Generates Fab Fragments of Cluster A Monoclonal Antibodies for Validation of Inner Domain of HIV-1 gp120?
Introduction
The lab of Dr. William D. Tolbert and Dr. Neelakshi Gohain use Papain-linked agarose resin to create combinations of purified Fabs of Cluster A monoclonal antibodies (A32 mAbs and JR4 mAbs) to validate the design and crystal structure of a stabilized inner domain (ID) of HIV-1 gp120. This ID displays a major antibody-dependent cellular cytotoxicity (ADCC) target of the A32 region. Current research supports a role for ADCC in preventing HIV-1 infection and in vaccine-induced protection that correlates with slower HIV progression or decreased virus replication.
Topics: Molecular Biology, Protein Estimation, Protein Concentration, Protein Labeling, Buffers & Chemicals
What is the Link between Protein Folding and Prion Disease?
Prion diseases (also referred to as transmissible spongiform encephalopathies, or TSEs) are a group of progressive, untreatable, and fatal neurodegenerative conditions affecting both humans and animals. Generally, these conditions are characterized by long incubation periods (usually between 5 to 20 years), disruption in the normal tissue structure (resulting in holes and vacuole formation in the neurons), and the absence of an inflammatory response.
Topics: Protein Purification, Molecular Biology, Western Blotting, Protein Electrophoresis, Cytotoxicity Assays, Sample Clean Up, Protein Concentration, Protein Fractionation, Protein Labeling, Protein Extraction, Buffers & Chemicals