Introduction
The lab of Dr. William D. Tolbert and Dr. Neelakshi Gohain use Papain-linked agarose resin to create combinations of purified Fabs of Cluster A monoclonal antibodies (A32 mAbs and JR4 mAbs) to validate the design and crystal structure of a stabilized inner domain (ID) of HIV-1 gp120. This ID displays a major antibody-dependent cellular cytotoxicity (ADCC) target of the A32 region. Current research supports a role for ADCC in preventing HIV-1 infection and in vaccine-induced protection that correlates with slower HIV progression or decreased virus replication.
They are the first to successfully isolate “the ID of HIV-1 gp120 as an independent protein molecule that efficiently encapsulates conformational A32-like epitopes within a minimal structural unit of the HIV-1 envelope without the complication of other known epitope specificities (Tolbert, W. D. et al, 2016).” Through structure-based design they developed the novel construct of Inner Domain 2 (ID2), which consists of the ID of the gp120 core stabilized in the CD4-bound conformation and expressed independently of the outer domain (OD) (Figure 1). CD4 is an antibody receptor protein found on the cell surface that contributes to ADCC protective immune function by exposing the cell’s A32-like entry targets to be bound by A32-like mAbs. These signals are natural killer (NK) cells to bind and release cytotoxic chemicals to kill the target cell. Thus, mAbs recognizing A32-like epitopes exhibit considerable ADCC potency and can eliminate infected cells, including cells budding HIV-1. Therefore, ID2 represents a novel probe for the analysis and/or selective induction of antibody responses to the A32 epitope region.
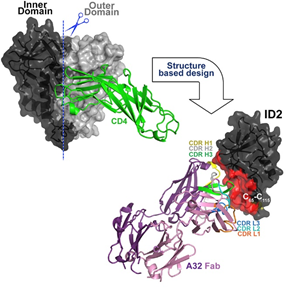
Figure 1. Novel Construct - A32 Fab-ID2
Construct Inner Domain 2 (ID2) stabilized in the CD4-bound conformation which consists of gp120 expressed independently of outer domain. ID2 displays the A32 ADCC epitope within a minimal structural unit of the HIV-1 Envelope.
Protocol for Fab Generations
The lab of Dr. Tolbert and Dr. Gohain used G-Biosciences’ Papain-linked agarose slurry to purify Fab fragments to create Fab-ID complexes for crystallization experiments. Papain is a cysteine protease enzyme (EC 3.4.22.2) that has the endopeptidase activity to cleave IgG molecules in the hinge region. This cleavage results in the generation of three ~50 kDa fragments; two Fab domains and a Fc domain (Figure 2).
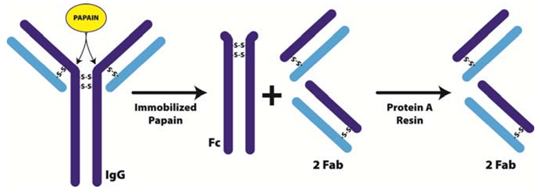
Figure 2. Immobilized Papain for the generation of Fab and Fc fragments from IgG
IgG molecule cleaved at the hinge regions by Papain’s endopeptidase activity. Cleavage results in the generation of three ~50 kDa fragments; two Fab domains and a Fc domain. The Fab domains are purified by running the digest through Protein A Resin which binds the IgG and Fc fragments. The papain‐digested antibody is unable to promote agglutination, precipitation, opsonization, and lysis.
The immobilized papain resin was equilibrated with a cysteine digestion buffer containing 20mM Cysteine. HCl at pH 7.0, by washing the resin twice with the cysteine digestion buffer. The IgG sample was cleaned with the supplied gel filtration columns (SpinOUT™) to remove all interfering agents and exchange the buffer to the cysteine digestion buffer for optimal digestion.
The optimized IgG sample was added to the equilibrated immobilized papain resin and incubated at 37°C with constant mixing for the manufacturer’s suggested digestion times. The digested antibody was collected by centrifugation and the papain was retained on the resin. The resin was washed to collect all the IgG and fragments.
Following papain digestion, the Fab fragments were separated from undigested IgG and the Fc region by passing the digest over the supplied protein A column. The column was equilibrated with PBS and then the digest was incubated with the Protein A resin for 10 minutes at room temperature with end-over-end mixing. The required Fab fragments were eluted by centrifugation and the whole IgG and Fc domains were retained on the resin. The resin was washed with PBS and the flow through collected for maximum Fab fragment recovery. The remaining Fc fragments and undigested IgG can be eluted by applying IgG elution buffer to the Protein A column and collecting the flow through, if required.
Results and Conclusions
Dr. Tolbert and Dr. Gohain successfully purified Fab fragments to generate Fab-ID complexes for crystallization experiments. These Fab-ID complexes were used to characterize ADCC inducing anti-Cluster A abs as possible vaccine targets against HIV.
The Fab-ID complexes were prepared by mixing purified Fab and ID protein at a 1:1.5 M ratio and incubating on ice for 30 min. The Fab-ID complexes were then purified by gel filtration chromatography. Crystals were grown by the hanging-drop method. Not all Fab-ID complexes (Cluster A mAbs A32, N5-iF, N60-I3, and JR4 complexed with ID1 and ID2) formed diffraction-quality crystals. Only Fab JR4-ID1 and Fab A32-ID2 complexes were selected for further analysis. Fab JR4-ID1 complex crystals were grown from 20% polyethylene glycol (PEG) monomethyl ether, 0.2 M ammonium sulfate, and 0.1 M Tris-HCl (pH 7.5). Fab A32-ID2 complex crystals were grown from 18% to 22% PEG 6000 or PEG 8000 and 0.1 M Tris-HCl (pH 8.5). JR4 and A32 complex crystals were flash-frozen in liquid nitrogen after a brief soak in the crystallization condition supplemented with 20% and 15% MPD, respectively. For structural analysis, diffraction data were collected at the Stanford Synchrotron Radiation Light Source. Structures were solved by molecular replacement with Phaser from the CCP4 suite (Tolbert, W. D. et al, 2016).
Their previous studies indicated that the ID undergoes substantial structural rearrangements for A32-like mAb recognition and is strictly dependent on binding cell surface anchored CD4. Structural analysis of the Fab JR4-ID1 complex revealed that the conformational CD4 epitopes of the A32-like region are not fully formed within ID1 and that large portions of the binding surface were disordered (Figure 3A). The calculated electron density maps showed clearly defined density for the whole JR4 Fab but for only 104 of 169 residues of ID1 (Table 1). These disordered regions around the ID1 V1V2 stem and α0 helix were flexible and did not contribute to anti-Cluster A mAb binding. They were able to correct this ID1 disorder by stabilizing the bases of the α0 and α1 helices with a disulfide bond which restored and preserved the CD4 bound conformation. Thus, they created ID2 with the V1V2 stem removed and stabilized by a C65-C115 disulfide bond (Figure 3B). ID2 was shown to have much higher binding affinities than ID1 (17-fold average) for anti-Cluster A mAbs which suggests that ID2 more closely resembles gp120 in the CD4-bound conformation.
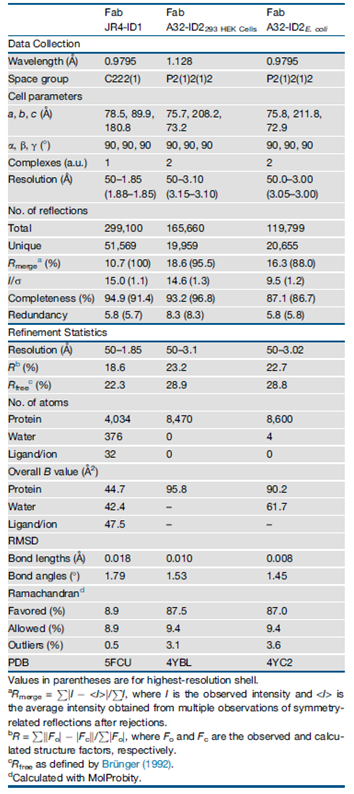
Table 1. Data Collection and Refinement Statistics
The crystal structure of ID2 in complex with mAb A32 Fab was determined to confirm whether the Cluster A epitopes of CD4-triggered gp120 were preserved better than ID1 (Figure 3B). ID2 fully preserved the fold of the ID of the gp120 core in its CD4-bound conformation. This includes the seven-stranded β sandwich and all the secondary structural elements of layers 1 and 2 (including α0 and α1 helices) present and arranged to form the C1-C2 epitope as seen in complexes of A32-like mAbs with CD4-triggered gp120 cores. Most of the ID2 molecule is defined in the complex (85% of the molecule, compared with 61% for ID1). The structural alignment of ID2 from the Fab A32-ID2 complex with the gp120 core and with ID1 resulted in a root-mean-square deviation (RMSD) between main-chain atoms of 0.76 Å (523 atoms) and 1.36 Å (416 atoms), respectively. This indicates a closer similarity of the overall structure of ID2 to the ID in the CD4-triggered gp120 cores bound to A32-like mAbs, than to ID1. The A32 Fab-ID2 complex represents the first co-crystal structure of mAb A32 with its cognate gp120 antigen. ID2 represents the first stable design of the gp120 ID expressed independently of the OD and stabilized in a CD4-bound confirmation to exclusively express the non-neutralizing, ADCC epitopes of the C1-C2 region (Tolbert, W. D. et al, 2016).
They found that ID2 stably engrafts the epitopes recognized by A32-like Abs involved in ADCC from the sera of HIV-1-infected individuals. These epitopes are involved in Fc-effector functions directed to the C1-C2 region of the HIV-1 Env. To test this, they performed a competition assay in which effector functions of anti-Cluster A mAbs were tested at their effective concentration in the presence of increasing concentrations of ID2. They found that ID2 completely inhibited all the A32-like mAbs tested which confirms that ID2 is folded to stably display functional A32-like epitopes within the ADCC Cluster A region. ID2 specifically bound Abs present in HIV+ sera and adsorbed approximately 30% of total ADCC activity. These results comply with previous observations indicating that A32 epitopes constitute a large fraction but not the only fraction of ADCC targets in sera of infected individuals (Tolbert, W. D. et al, 2016).
Dr. Tolbert and Dr. Gohain are the first to define the structure of the mAb A32 epitope, the canonical mAb of the Cluster A region. The ADCC responses specific for the Cluster A epitope are highly conserved among HIV isolates. Therefore, they represent an important vaccine target. Thus, ID2 can potentially be used to elicit anti-Cluster A Abs in vivo as a minimal structural unit of gp120 effectively expressing the A32-like region. ID2 has significant translational value because it can be employed as a specific probe for the analysis of Ab responses to these non-neutralizing epitope targets (e.g., in immune correlates analysis of future vaccine trials or epitope mapping). It also constitutes a novel immunogen candidate selectively and stably presenting the ADCC A32-like epitopes that could be used in challenge studies to address the exclusive role of non-neutralizing Abs in vaccine protection (Tolbert, W. D. et al, 2016).
The Fab A32-ID2 complex represents the first co-crystal structure of mAb A32 with its gp120 antigen and permits the precise description of the gp120-binding footprint of mAb A32. mAb A32 recognizes the same discontinuous site within the C1-C2 region as other anti-Cluster A mAbs that are capable of potent FcR-effector function against virus bound targets. The A32 epitope is buried inside the HIV-1 Env trimer and is not accessible unless bound by cell surface CD4. The HIV-1 accessory viral protein U and negative regulatory factor protein reduce the levels of CD4 on the surface of the infected target. Consequently, the HIV-1 Env trimers remain closed and inhibit the recognition of infected cells by mAb A32. Therefore, the potential utilization of mAb A32 and other Abs targeting A32 region epitopes to inactivate HIV infected cells will be possible only if CD4-downregulation can be effectively diminished.
References
Tolbert, W. D. et al (2016) Paring Down HIV Env: Design and Crystal Structure of a Stabilized Inner Domain of HIV-1 gp120 Displaying a Major ADCC Target of the A32 Region. Journal/ Year/ Vol #: Structure/2016/24
Related G-Biosciences Products
|
Catalog# |
Product Name |
|
786-790 |
Immobilized Papain |
|
786-272 |
Fab Fragmentation (Macro) Kit |
|
786-273 |
Fab Fragmentation (Micro) Kit |
|
786-283 |
Protein A Resin, 5ml |
|
786-824 |
Protein A Resin, 25ml |
|
786-825 |
Protein A Resin, 5 x 1ml Columns |
|
786-826 |
Protein A Resin, 5 Column Kit |
|
786-827 |
Protein A Resin, 10 x 0.2ml Columns |
|
786-828 |
Protein A Resin, 10 Column Kit |