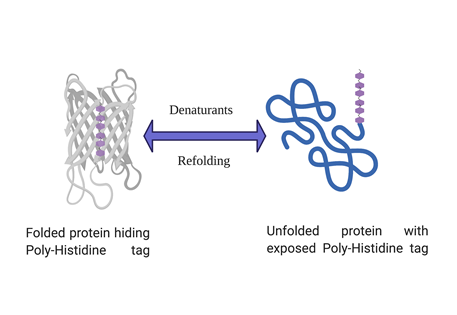
Poly-histidine tagged proteins account for more than 90% of routine recombinant protein expression and purification. Histidine tag has many advantages over other affinity tags such as small size, high affinity for binding to IMAC resins (Immobilized metal affinity chromatography) and allows one step purification of recombinant protein. But sometimes a histidine tagged protein doesn’t bind to the affinity column and evades it’s capture and subsequent purification. Although such instances are not very common but can be very frustrating whenever they happen. However, there are ways to troubleshoot if such a problem arises and the solutions are discussed below in a step by step manner.
Aaron McCoy
Recent Posts
His-tagged protein expressing but not binding your Ni-NTA column?
Posted by
Aaron McCoy on Dec 23, 2020 1:00:00 PM
0 Comments Click here to read/write comments
Topics: Protein Purification, Western Blotting, Protein Electrophoresis, Sample Clean Up, Protein Concentration, Protein Fractionation, Protein Labeling, Protein Extraction, Protein Detection