The p53 gene is a tumor repressor gene responsible for regulating cell replication cycle to prevent them from growing and proliferating too fast. Additionally, it also induces programmed cell death (apoptosis) in irreparable cells to inhibit the development, growth, and proliferation of tumor cells.
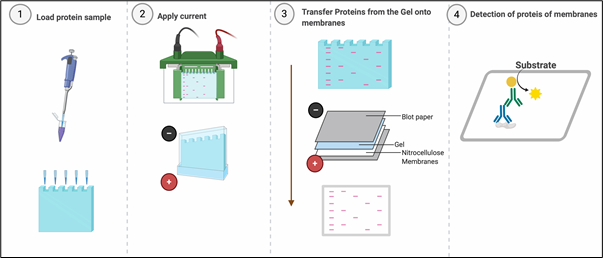
Western blotting or immunoblotting is an indispensable technique, almost every published paper in area of molecular cell biology uses western blotting for detecting specific proteins in a sample of tissue homogenate or cell lysates. Western blotting combines resolving power of polyacrylamide gel electrophoresis (PAGE) or SDS-PAGE and specificity of antibodies to detect target proteins. Proteins are resolved on the basis of their molecular weight in SDS-PAGE and transferred from the polyacrylamide gel onto the membranes (Nitrocellulose or PVDF), which creates an exact replica of the protein separation pattern on the membrane. After transferring the proteins to the membrane, the membrane needs ‘blocking’ to ‘block’ non-specific binding sites on the surface of the membrane. Blocking is usually performed with Bovine Serum Albumin, Skimmed milk or purified milk Casein.
Topics: Protein Purification, Western Blotting, Protein Electrophoresis, Protein Estimation, Sample Clean Up, Protease Inhibitors, Protein Extraction, Protein Detection