Cysteine is one of the most reactive amino acid residue owing to the thiol (-SH) group, thiols can serve as an electron donor in different reactions. Cysteine thiols can form part of catalytic centre and can frequently participate in enzymatic reactions, not just that, cysteine thiols can also undergo a variety of covalent post-translational modifications (PTMs) and formation of disulphide bonds. The thiol modifications can act as important mediators of redox signalling and protein function regulation.
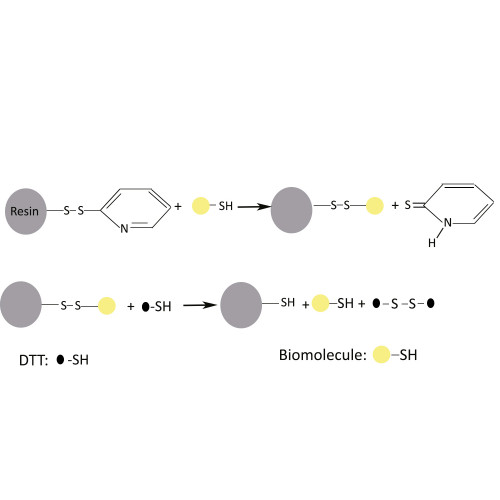
Cysteine residues can undergo a variety of post translational modifications such as nitrosylation, palmitoylation and glutathionylation. These thiol modifications are reversible and play important roles in normal and pathophysiological processes. Thiopropyl resin can be used to capture proteins containing reversible cysteine thiol modifications. Thiopropyl resin works on the principle of covalent chromatography, it forms mixed disulphides with the proteins containing thiols, by exchanging the labile Thiopropyl resin contains reactive 2-thiopyridyl disulphide group attached to resin through a chemically stable ether linkage.
Thiopropyl resin can be used to enrich and purify the proteins containing reversible cysteine modifications. The proteome sample is treated with a thiol reactive blocking agent (such as N-ethylmaleimide so as to block all of the unmodified free cysteines. Once the free thiols have been blocked the proteome samples can be then treated with reducing agents so as to bring about selective reduction of cysteines bearing reversible modifications. The selective reduction is achieved by using different reducing reagents that react specifically with each kind of cysteine modification (e.g., ascorbate for S-Nitrosylation, Hydroxylamine for Palmitoylation, Glutaredoxin for S-Glutathionylation). Finally the regeneration of nascent thiol concomitant with selective reduction is necessary for capture of such proteins on Thiopropyl resin. The nascent thiols enable formation of mixed disulphides with the thiopropyl resin exchanging thiopyridyl group.
Once the proteins have been captured on the resin they can be they subjected to
on-resin protein digestion and multiplexed isobaric labelling to facilitate liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based quantitative site-specific analysis of cysteine-based reversible modifications.
Some recommendation that we make for use of the resin are given below.
Binding buffer: 50mM HEPES buffer or 20 mM Tris-HCl, 0.1–0.5 M NaCl, pH 7.0.
To facilitate exposure of all the thiols, use of 2% SDS (recommended) or 8 M urea or 6 M guanidine HCl can be done. This ensures that the proteins are denatured and all thiol groups are accessible for the reaction.
Elution buffer alternatives:
For covalently bound proteins we recommend 0.025 M cysteine, 50 mM Tris-HCl, pH 7–8.
To minimize reduction of intramolecular disulphide bridges: 5–20 mM L-cysteine, 50 mM Tris-HCl, 1 mM EDTA, pH 8.0 or 20–50 mM 2-mercaptoethanol, 50 mM Tris-HCl, 1 mM EDTA, pH 8.0.
Important: When using Thiopropyl Resin, 2-thiopyridyl groups must be removed after the protein has bound. To achieve this column can be washed with sodium acetate 0.1 M, 2-mercaptoethanol 5 mM, pH 4.0 before beginning elution.
We recommend degassing all buffers to avoid oxidation of free thiol groups.
It is highly recommended to use preliminary titration studies with 2,2’-dipyridyl disulphides to determine optimal binding conditions before proceeding with main experiment. The release 2-thiopyridone can be determined spectrophotometrically (absorbance coefficient = 8.08 x 103 M-1 cm-1 at 343 nm) when the sample (1–5 mg in 1–3 ml binding buffer) reacts with 2, 2’-dipyridyl disulphides. Under standard conditions at pH 7.5, a few minutes are usually enough for the completion of the reaction.