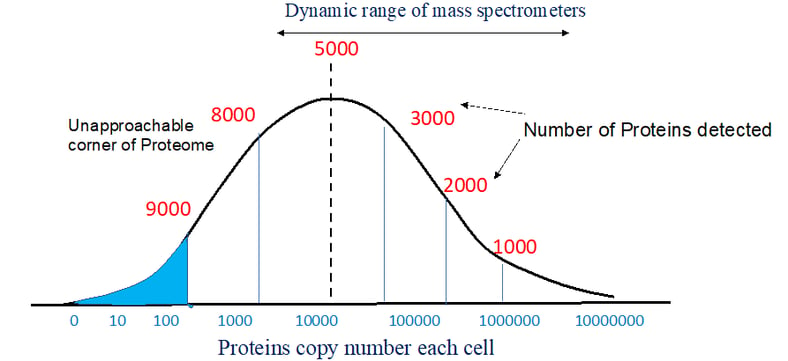
A proteome is a sum total of all the cellular proteins, this would include housekeeping proteins and proteins that are produced in response to a stimulus. Some proteins are produced only in a given cell type or tissue, others are produced in all cells, some proteins are only produced under certain given conditions adding to the complexity and heterogeneity of proteins in a proteome. Similarly, alternative splicing and post translational modifications all together combined can make the proteome very complex.
Capture and purification of low abundance proteins
Topics: Protein Purification, Protein Concentration, Peptide Generation
How to Get Accurate Results with Affinity Purification and Affinity Chromatography
There are various methods to enrich or purify proteins of interest from other proteins and components. Most purification methods involve a form of chromatography where molecules in a solution or mobile phase separate based on differences in chemical or physical interaction with a stationary material or solid phase.
Topics: Protein Purification
Purify Proteins Fast: How FPLC Speeds the Process
Many researchers use high pressure liquid chromatography (HPLC) to separate and measure concentrations of small molecules. However, this method is relatively ineffective when trying to extract proteins and other biochemicals. Organic solvents used in HPLC place restrictions on these biochemicals, which are unable to function at high temperatures. For this reason, in 1982, Pharmacia developed fast protein liquid chromatography (FPLC) to overcome the weaknesses of the HPLC and institute an effective, quick means to extract and purify biochemicals - especially proteins.
Topics: Protein Purification
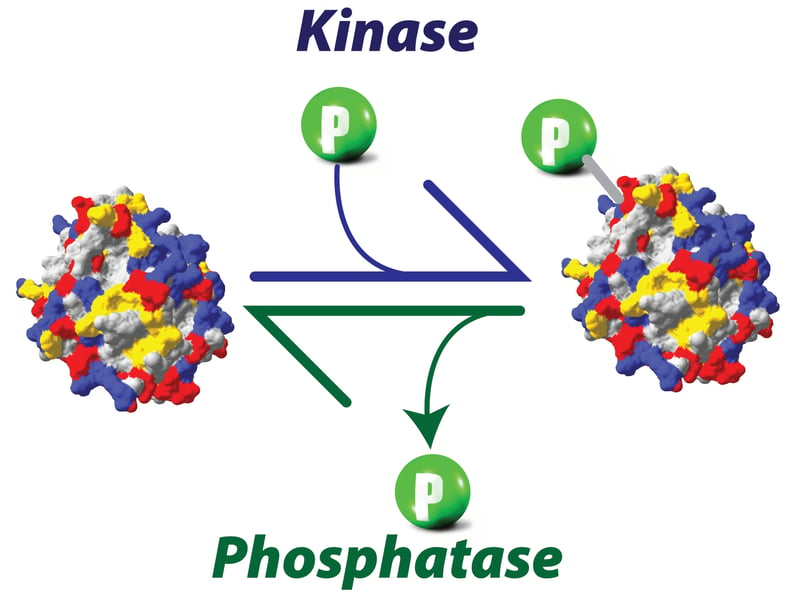
Protein phosphorylation: Nature’s favorite way of decorating proteins
Part 1: Essentials of protein phosphorylation
American chemist Westheimer asked an interesting question in 1987 in his very famous article published in ‘Science’ the same year, ‘why nature chose phosphates?’. He elegantly approached this problem from a chemists’ perspective. Phosphorus, a group 15 element, is an essential element of biomolecules. Phosphorus and its chemistry pervade cells and living systems, ATP, the ubiquitous currency of energy, is a nucleotide with two phosphates available in high energy phospho anhydride bonds. The backbone of genetic material (DNA or RNA) is made up of phosphates, two adjacent nucleotides are linked by phosphodiester bonds. The negative charge present on phosphodiester linkages in nucleic acids makes them resistant to hydrolysis and therefore very stable in aqueous environments, a key feature required for a molecule to qualify as a genetic material is chemical stability. Other group 15 elements like arsenic don’t make esters that are stable in aqueous conditions at neutral pH.
Topics: Protein Purification, Protein Labeling