There are various methods to enrich or purify proteins of interest from other proteins and components. Most purification methods involve a form of chromatography where molecules in a solution or mobile phase separate based on differences in chemical or physical interaction with a stationary material or solid phase.
The most powerful method is affinity purification, where the protein of interest purifies and rids itself of contaminating proteins by its specific binding properties to an immobilized ligand.
What is Affinity Purification?
Affinity purification, otherwise known as affinity chromatography, is a laboratory technique that purifies proteins or protein complexes within a biochemical mixture.
Unlike other purification methods such as gel filtration and size-exclusion chromatography, affinity chromatography manipulates specific molecular properties and binding interactions between molecules to purify the protein of interest.
In specific, a particular ligand chemically immobilizes to a solid support, so when a complex mixture passed over the column, the molecules with specific binding affinity bind to the ligand.
After other sample components wash away, the bound molecule separates from the support, which results in its purification from the original sample.
Affinity Purification Applications
There are multiple techniques for affinity chromatography, including:
- Analytical affinity chromatography
- Antibody purification
- Antigen purification using antibodies
- Boronate affinity chromatography
- Dye-ligand affinity chromatography
- Immunoaffinity or the purification of antibodies from blood serum
- Immobilized metal ion affinity chromatography (IMAC)
- Lectin affinity chromatography
- Purification of recombinant proteins
How Does Affinity Purification Work?
Affinity chromatography works by manipulating the interaction strength differences between biomolecules in both the mobile and stationary phases.
As a complex mixture passes over a solid support column, the ligand that is bound to the column interacts with the molecules that have a specific binding affinity to the particular ligand. As a result, the protein of interest binds with the immobilized molecules.
Generally, there are three steps to follow to achieve affinity chromatography:
- Incubate the crude sample (cell lysate, serum, growth medium) or mobile phase with the affinity support or stationary phase (typically an agarose gel matrix or other porous resin), so the target molecule in the sample binds to the immobilized ligand.
- Elute any non-bound sample components from the support by using appropriate buffers that maintain the binding interaction between target and ligand. Since the target molecules have a stronger affinity for the stationary phase, only the non-target molecules will wash away.
- Release the target molecule from the immobilized molecule by using an elution buffer with a higher salt concentration, which results in the recovery of a highly purified and concentrated material.
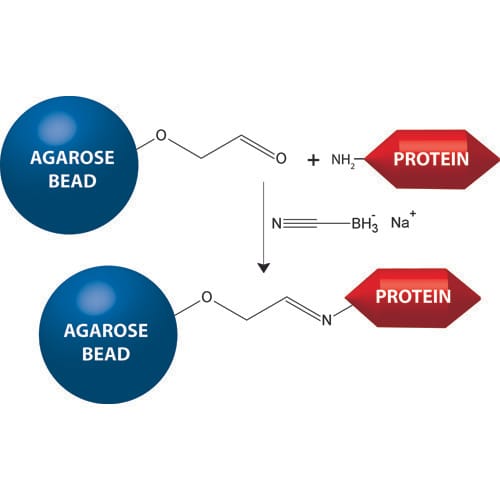
Note: There are two ways to immobilize ligands to the solid support material. It can either be through the formation of covalent bonds between particular functional groups and the reactive groups in the support, or through indirect coupling methods.
How to Get Accurate Results with Affinity Purification
To achieve accurate results with affinity chromatography, consider the following tips:
- Thoroughly wash the affinity medium before use, to remove all traces of storage solutions and preservatives.
- Always use high-quality solutions and preservatives.
- Avoid reusing affinity media unless there are identical samples.
- Magnetic stirrers can damage the matrix, so use mild rotation or end-over-end stirring instead.
- Test the affinity of the ligand-target molecule interaction when possible since extremely low or high affinity significantly reduces the yield.
- Adjust the sample to the composition and pH of the binding buffer to improve the binding efficiency of the target protein.
- Use high flow rates for interactions with strong ligand-target molecule affinity but use a lower flow rate for interactions with weak affinity or slow equilibrium.
Choose G-Biosciences for Your Affinity Purification Needs
At G-Biosciences, we offer a selection of products for affinity chromatography. To shop our selection, visit our website and contact us with any questions.