Proteins that form complexes tend to form aggregates during recombinant expression. So the best method is to co-express proteins with suitable partner protein. Chaperones are one of those proteins that help insoluble protein formation. Researchers observed the enhanced solubility of some proteins when co-expressed with chaperones. However, the choice of suitable chaperone for a particular protein is difficult to assume and only with trial and error methods one can achieve a soluble protein. Chaperones act as catalysts that prevent aggregation of nascent chains and unfolding aggregate proteins. Co-expression of recombinant proteins with chaperones help in improved solubility, enhanced specific activity and yield of target proteins.
Use of Chaperones in Recombinant Protein Expression
Topics: Protein Purification
Protein purification based on fusing peptide affinity tag to recombinant protein is widely preferred due to its ease of use. Immobilized Metal Ion Affinity Chromatography (IMAC) was based on the principle of binding of specific amino acid side chains of a protein to metal ions immobilized on a matrix. Histidine amino acid has high affinity to metal ions, six histidine residues are fused to N-terminal or C-terminal end of a protein during cloning, and the protein obtained could be ~95% pure after passing through metal ion based resin. Protein purification based on metal affinity chromatography is used with all the expression systems including E.coli, Yeast, Insect cell and mammalian cell expression systems.
Topics: Protein Purification
Detergent Screening For Membrane Protein Extraction: What To Choose?
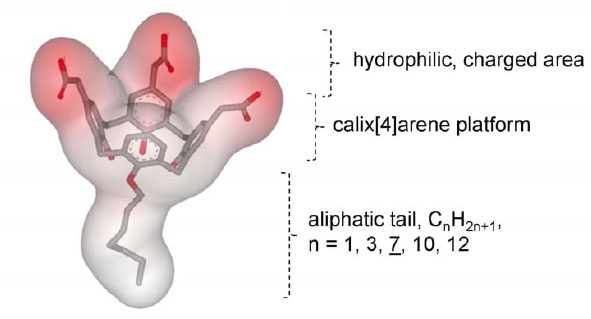
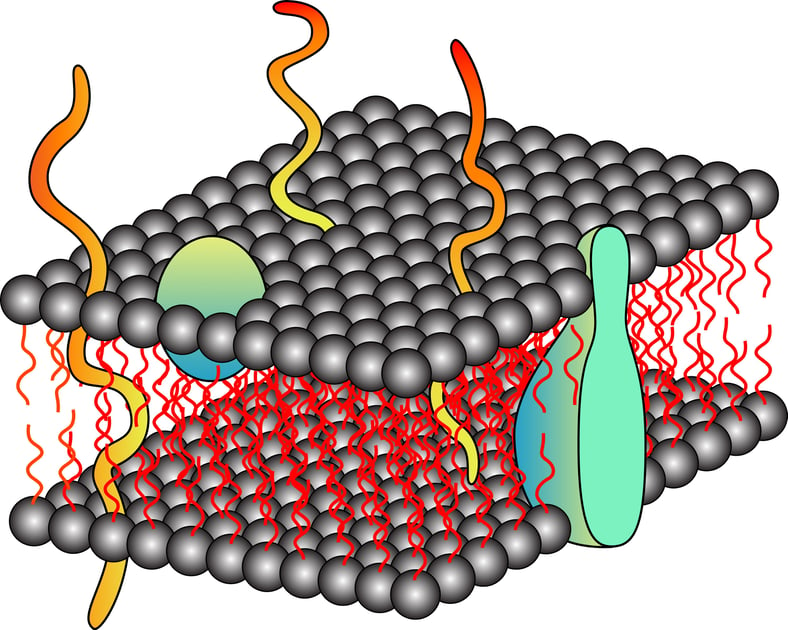
With the advancement of biochemical tools, studies on membrane proteins have grown substantially. Since membrane proteins are difficult to extract and purify, it requires optimization and an orchestrated execution. As described in our basics of membrane protein isolation blog, surface active agents are crucial for the manipulation of membrane proteins. The amphiphillic properties of these components promote the interaction of the membrane proteins, which are wrapped around by hydrophobic lipid bilayer in their native state, to become water soluble. Nonetheless, the solubility cannot be extrapolated into stability and restoration of the native functional structure; therefore, it is not necessary that a detergent (surfactant or a surface-active agent) can yield a suitable stable membrane protein fraction despite good extraction. Also, a detergent that has shown good results previously with a particular membrane protein might not work well with other membrane proteins. In the absence of gold standards or thumb rules for membrane protein extraction, it becomes imperative to understand the physiochemical characteristics of different detergents before extracting the proteins for example, the charge and degree of hydrophobicity of a specific detergent can allow a prediction of its behavior in a solution and interaction with the protein of choice.
Topics: Protein Purification, Detergents, Protein Extraction
Proteins are the building blocks of cells and are the most abundant and diverse biomolecules. Proteins are literally responsible for almost all the functional aspect of a cell, from making the cellular membrane to catalyzing a biological reaction. As per the database, approximately 20-30% of the total encoded proteins have integral membrane proteins while 10-20% portion comprises membrane-associated proteins. This diverse group of proteins includes signal transducers, transporters, membrane channels, and cell surface receptors. This specialized group of proteins has become a target for therapeutic drug development in the past few decades. However, due to the unavailability of complete structural details of these proteins, targeting is a little difficult and that makes drug designing trickier than expected. The right approach is to design polished methods that can help the extraction of membrane proteins and their analysis.
Topics: Protein Purification, Detergents, Protein Extraction