In a cell, protein degradation and synthesis maintain the balance of proteins, where old proteins are degraded into amino acids, which are further used in the synthesis of new proteins. Proteases/Peptidases play an important role in protein degradation. Proteases are present in almost all living cells. In a living cell, protease action is regulated by external as well as internal factors. Some proteases are inactive under normal conditions, but are activated only when necessary. For example, during apoptosis, Pro-caspase (inactive) converts to Caspase (active). Sub-cellular localization of proteases also helps in their regulated activity. Most of them are localized to sub-cellular organelles like lysosomes and thereby restricted their activity only to those proteins directed to lysosomes.
0 Comments Click here to read/write comments
Topics: Protease Inhibitors
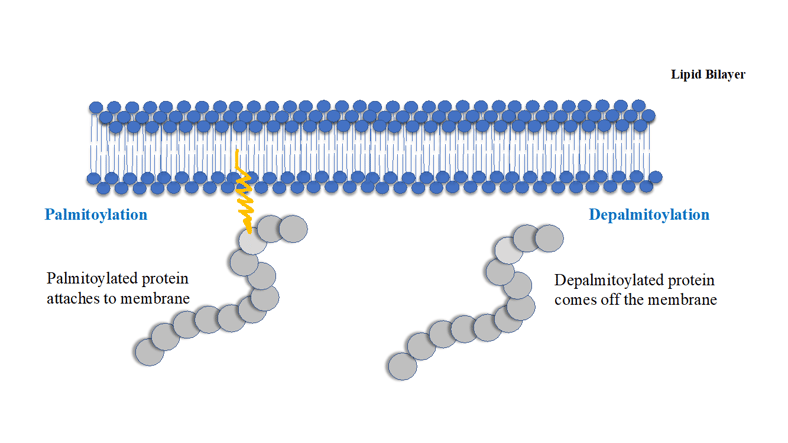
Protein palmitoylation and sulfhydryl chemistry methods to capture palmitoylated proteins
Posted by
The Protein Man on Oct 29, 2019 2:30:00 PM
Proteins undergo many kinds of post translational modifications (PTMs), such as phosphorylation, ubiquitination, SUMOylation, geranyl-geranylation, farnesylation, myristoylation, acetylation, succinylation and palmitoylation. PTMs can be reversible or irreversible, they can be dynamic or stable. PTMs enable fine tuning of protein function in response to various internal and external signals and therefore they have profound effects on cellular physiology, metabolism, survival and growth.
0 Comments Click here to read/write comments
Topics: Protein Labeling
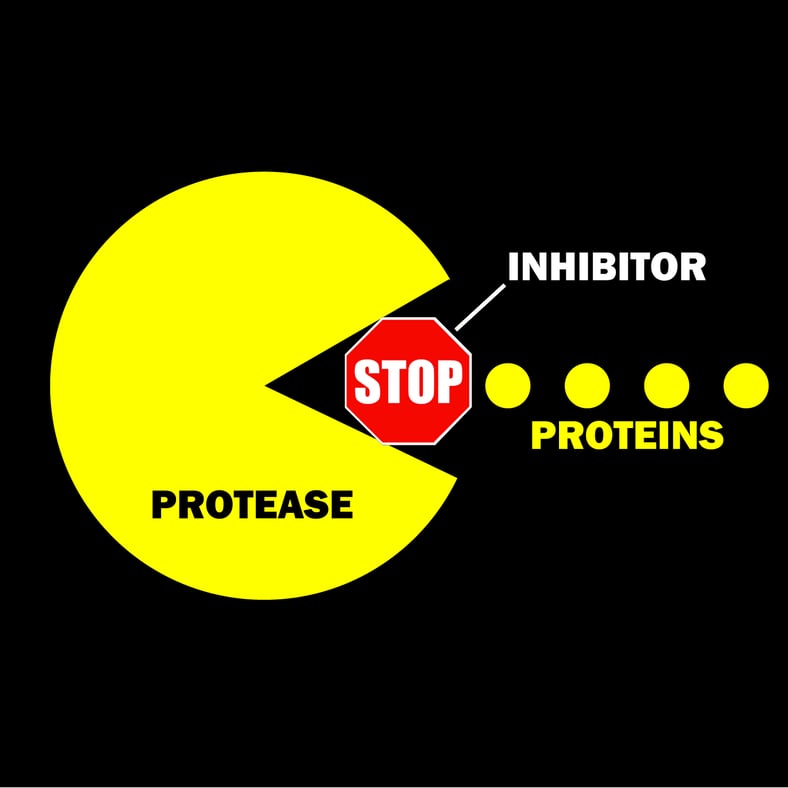
Complete Protease Inhibitor Cocktails and How They Work
Posted by
The Protein Man on Oct 22, 2019 2:30:00 PM
What is a Protease Inhibitor?
If you want to protect your target protein from potential harm, there is one thing you should do – use a protease inhibitor.
0 Comments Click here to read/write comments
Topics: Protease Inhibitors
The Difference Between the BCA and Bradford Protein Assays
Posted by
The Protein Man on Oct 15, 2019 2:30:00 PM
0 Comments Click here to read/write comments
Topics: Protein Estimation