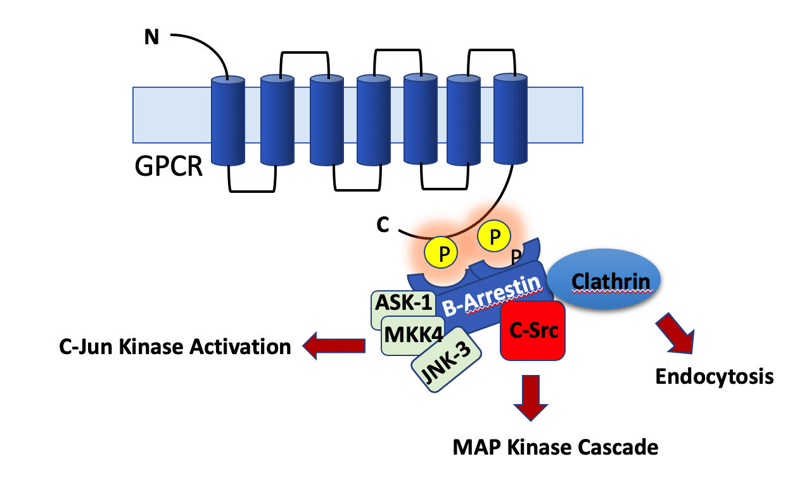
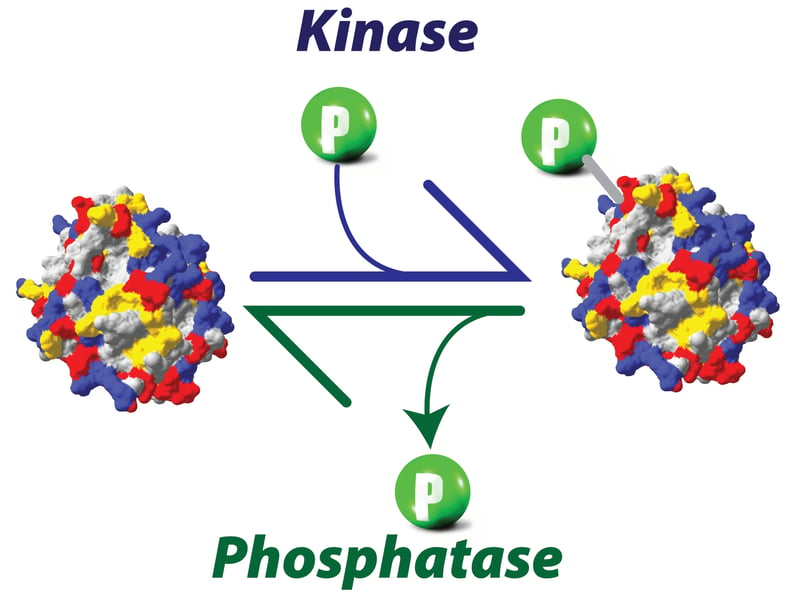
Proteins are phosphorylated and dephosphorylated all the times in the cells in a highly regulated manner. Protein phosphorylation and dephosphorylation are critical for signaling, cell division, protein translation, metabolism and survival. Activity of substrate proteins is tightly regulated by concerted activities of kinases and phosphatases in-vivo. On an average, roughly 3% of a typical eukaryotic genome encodes for kinases or phosphatases, yet more than 30% of the total number of proteins in a cell undergoes phosphorylation. As previously discussed, there is no general rule about effect of phosphorylation on proteins and same is true for dephosphorylation also. The effect of phosphorylation or dephosphorylation varies in context with different proteins.
Protein Kinases and Phosphatases: drivers of phosphorylation and dephosphorylation
Topics: Protease Inhibitors
Protein phosphorylation: Nature’s favorite way of decorating proteins
Part 1: Essentials of protein phosphorylation
American chemist Westheimer asked an interesting question in 1987 in his very famous article published in ‘Science’ the same year, ‘why nature chose phosphates?’. He elegantly approached this problem from a chemists’ perspective. Phosphorus, a group 15 element, is an essential element of biomolecules. Phosphorus and its chemistry pervade cells and living systems, ATP, the ubiquitous currency of energy, is a nucleotide with two phosphates available in high energy phospho anhydride bonds. The backbone of genetic material (DNA or RNA) is made up of phosphates, two adjacent nucleotides are linked by phosphodiester bonds. The negative charge present on phosphodiester linkages in nucleic acids makes them resistant to hydrolysis and therefore very stable in aqueous environments, a key feature required for a molecule to qualify as a genetic material is chemical stability. Other group 15 elements like arsenic don’t make esters that are stable in aqueous conditions at neutral pH.
Topics: Protein Purification, Protein Labeling
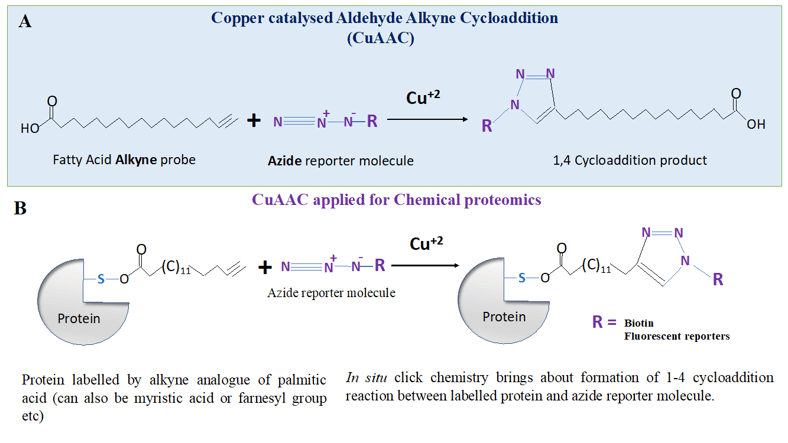
Click chemistry and its application to proteomics
Nature has ability to generate an unending array of molecules from very small number of building blocks, even simple microbes such as E. coli have synthetic chemical prowess that can bring the most sophisticated organic chemistry labs to their shame. Inspired by the nature’s ability to knit complex molecules from a small number of building blocks, using simple modular reactions, Berry Sharpless, and his coworkers, defined and invented the area of ‘click chemistry’. Together, Berry Sharpless and M. G Finn redefined the use of simple modular reactions that had already existed to generate new ‘functions’ or properties and termed it as ‘click chemistry’.
Topics: Protein Labeling
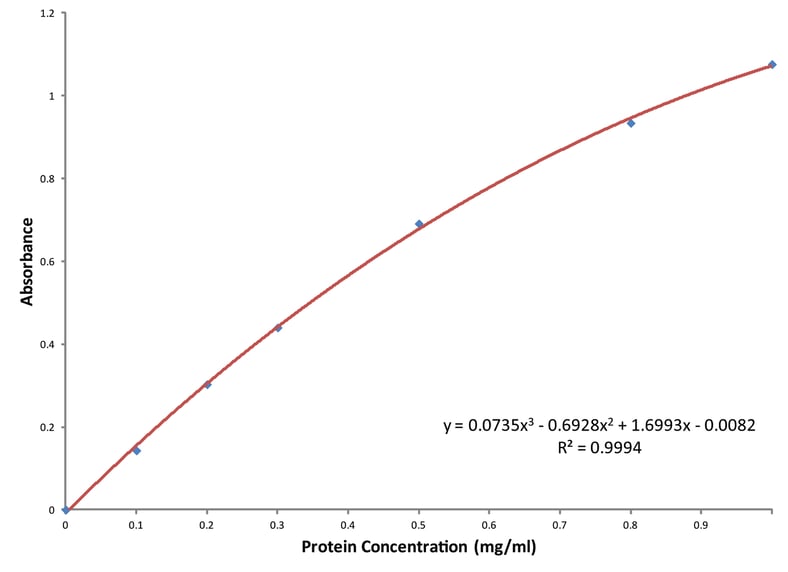
Why Is Bovine Serum the Preferred Standard for Protein Assays?
Protein assays are one of the most widely used scientific methods, particularly in life sciences. An assay is an efficient spectroscopic procedure that analyzes the concentration of protein in a solution, also referred to as protein concentration quantitation. Being able to measure this concentration is an integral part of any laboratory workflow involving protein extraction, purification, labeling, or analysis. It is necessary for an array of research, including processing protein samples for isolation, separation, and analysis by chromatographic, electrophoretic, and immunochemical techniques. There are different types of assays and each can be useful for different applications, but many assays work in similar ways and utilize standards.
Topics: Protein Estimation