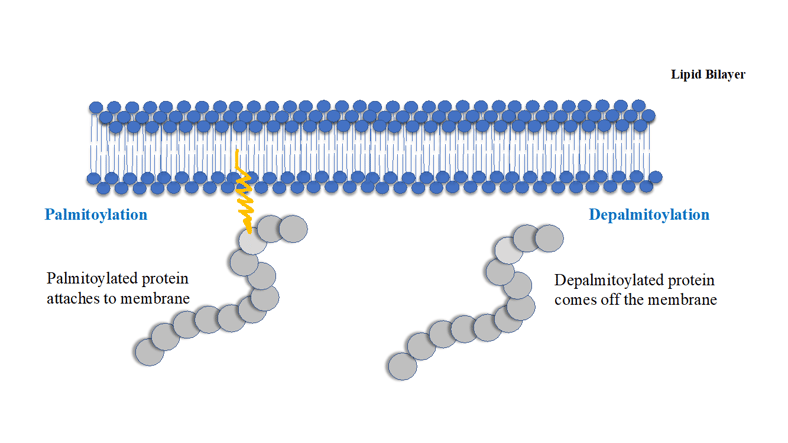
Proteins undergo many kinds of post translational modifications (PTMs), such as phosphorylation, ubiquitination, SUMOylation, geranyl-geranylation, farnesylation, myristoylation, acetylation, succinylation and palmitoylation. PTMs can be reversible or irreversible, they can be dynamic or stable. PTMs enable fine tuning of protein function in response to various internal and external signals and therefore they have profound effects on cellular physiology, metabolism, survival and growth.
The Protein Man

Recent Posts
Protein palmitoylation and sulfhydryl chemistry methods to capture palmitoylated proteins
Topics: Protein Labeling
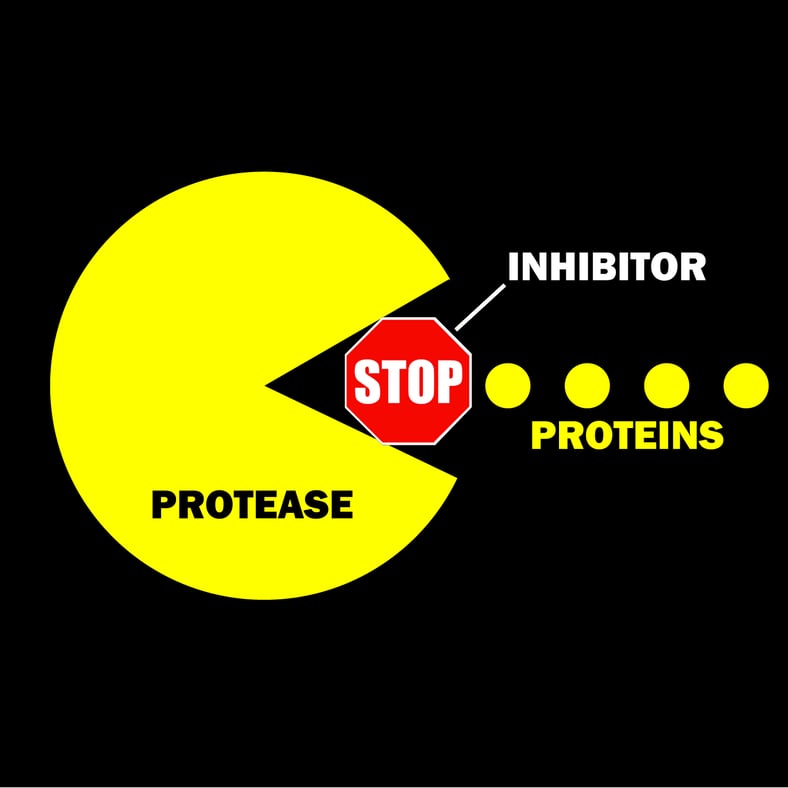
Complete Protease Inhibitor Cocktails and How They Work
What is a Protease Inhibitor?
If you want to protect your target protein from potential harm, there is one thing you should do – use a protease inhibitor.
Topics: Protease Inhibitors
The Difference Between the BCA and Bradford Protein Assays
Topics: Protein Estimation
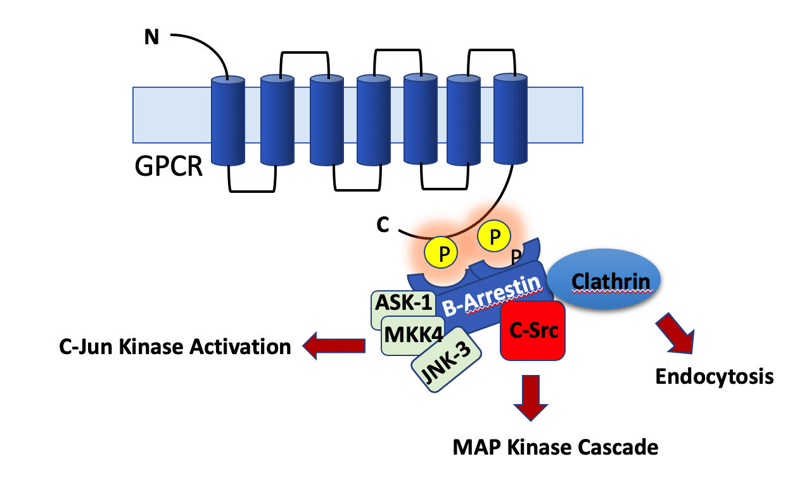
Protein Kinases and Phosphatases: drivers of phosphorylation and dephosphorylation
Proteins are phosphorylated and dephosphorylated all the times in the cells in a highly regulated manner. Protein phosphorylation and dephosphorylation are critical for signaling, cell division, protein translation, metabolism and survival. Activity of substrate proteins is tightly regulated by concerted activities of kinases and phosphatases in-vivo. On an average, roughly 3% of a typical eukaryotic genome encodes for kinases or phosphatases, yet more than 30% of the total number of proteins in a cell undergoes phosphorylation. As previously discussed, there is no general rule about effect of phosphorylation on proteins and same is true for dephosphorylation also. The effect of phosphorylation or dephosphorylation varies in context with different proteins.
Topics: Protease Inhibitors