With the advancement of biochemical tools, studies on membrane proteins have grown substantially. Since membrane proteins are difficult to extract and purify, it requires optimization and an orchestrated execution. As described in our basics of membrane protein isolation blog, surface active agents are crucial for the manipulation of membrane proteins. The amphiphillic properties of these components promote the interaction of the membrane proteins, which are wrapped around by hydrophobic lipid bilayer in their native state, to become water soluble. Nonetheless, the solubility cannot be extrapolated into stability and restoration of the native functional structure; therefore, it is not necessary that a detergent (surfactant or a surface-active agent) can yield a suitable stable membrane protein fraction despite good extraction. Also, a detergent that has shown good results previously with a particular membrane protein might not work well with other membrane proteins. In the absence of gold standards or thumb rules for membrane protein extraction, it becomes imperative to understand the physiochemical characteristics of different detergents before extracting the proteins for example, the charge and degree of hydrophobicity of a specific detergent can allow a prediction of its behavior in a solution and interaction with the protein of choice.
Extraction of the membrane proteins from the lipid bilayer is facilitated by detergents. The detergent makes the proteins partially soluble in the buffer by making a protein: detergent complex. However, the more the stringency of the detergent, higher is the probability of irreversible protein denaturation. Therefore, by rule, it is suggested to use mild detergent so that, the basic structure of the protein and its native conformation can be retained in the soluble state.
Practical issues to be addressed while working with membrane proteins:
- The detergent to be used should be of higher purity level and homogenous in solution. It should be devoid of contaminants such as amides, alcohols or any other synthetic molecules. A high-grade detergent should always be preferred.
- While quantifying the extracted protein, aggregate molecular weight should be considered for the detergent: protein complex such as, for a small protein (smaller size than the micelle), the proportion is generally 1:1. In that case, the aggregate weight= molecular weight of the protein+ aggregate weight of the micelle. Contrary to that, the micelle size and the detergent requirement for a large membrane protein are higher.
These days, surfactants that are composed of fluorocarbon present better alternatives than the conventional hydrocarbon-based detergents. However, the usefulness of these unconventional surfactants varies considerably, which will be reviewed in this article further.
Nanodiscs
Nanodiscs are closely similar to a detergent micelle in structure. It consists of an aggregation of 130-160 lipids arranged in a bilayer manner held together by amphipathic proteins known as Membrane scaffolding proteins (MSPs). This allows a greater advantage over detergents in terms of stability and solubility. In this approach, the targeted proteins are transiently solubilized using a mild detergent and nanodiscs. The detergent is removed for further procedure, leaving the targeted membrane protein enclosed within the nanodisc assembly. This arrangement mimics a native-state like condition and allows much more stability at a single molecule level.
This method is advantageous for the extraction of transmembrane proteins. However, it needs optimization at the level of detergent used in combination with nanodiscs as the very first step.
Fluorinated Surfactants (FS)
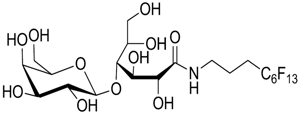 Fluorinated surfactants do not have detergent properties and they cannot penetrate well into the lipid bilayer. However, they work efficiently in combination with conventional detergents and in the later stages; they can easily replace normal detergents for solubilizing the membrane protein.
Fluorinated surfactants do not have detergent properties and they cannot penetrate well into the lipid bilayer. However, they work efficiently in combination with conventional detergents and in the later stages; they can easily replace normal detergents for solubilizing the membrane protein.
Structurally, FSs are closely similar to the classical detergents except for the presence of fluorine atoms in the hydrophobic tails. Incorporation of fluorine atoms allow bulkiness and rigidity to the overall structure and also contribute to having duality in terms of physiochemical attributes. FSs have both hydrophobic and lipophobic characteristics which make them inert and less permeable to the lipid bilayer. Their inertness provides stability to the extracted protein as the bulky fluorinated tails are largely unable to disrupt the interior of the protein. Compared to conventional surfactants, FSs yield lower critical micelle concentration (CMC) and help in preserving the structural and functional properties of the extracted membrane proteins.
Another variation of fluorinated surfactants is additional hydrogenation at the fluorinated tails by the incorporation of an ethyl or propyl group, generating HEMIFLUORINATED SURFACTANTS. These surfactants have additional advantages to improve protein-protein interaction and reducing membrane protein aggregation.
As non-specific aggregations are easily avoided with Fluorinated and hemifluorinated surfactants along with improved crystallization, therefore, these surfactants are good for cryo-EM.
Poly(tris) and Lactobionamide Surfactants
These non-ionic surfactants are derived from the modification of a buffer, tris(hydroxymethyl)aminomethane having either hydrogenated or fluorinated hydrophobic chains (monodisperse headgroup).
Lactose-derived Lactobionamide surfactants have a polar head group, similar to n-dodecyl-β-D-maltoside (DDM), a commonly used detergent. However an additional fluorinated or hemifluorinated tail allows them to stabilize very delicate and fragile protein complexes.
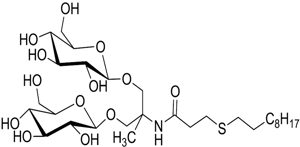 bis-Glucose Surfactants
bis-Glucose Surfactants
These sufactants carry a tetrahedral chiral carbon having two polar headgroups of glucose moieties and a long hydrophobic tail. This allows a long, cylindrical micellar structure formation and makes a stabilizing core for membrane proteins.
Calixarene surfactants
These are a new class of anionic detergents developed very recently for the stabilization of extracted and purified membrane proteins. Calixarene-based detergents have shown greater possibilities in the field of Membrane protein characterization. They have shown a promising role in mimicking native bilayer membrane environment and the comparative rigidity of the membrane protein and the surrounding lipids. These surfactants have mild stringency and low CMCs (0.05-1.5 mM) with a small micelle structure (5-24 nm). Calixarene surfactants interact with membrane proteins via the positively charged residues present on the membrane proteins. This interaction results in a favorable packing of the calixarene molecules with the membrane proteins even in the absence of a lipid environment. The conformation and stability of the proteins stay intact all throughout. Different modifications of Calixarene are available these days to enhance protein stability and facilitate efficient protein purification.
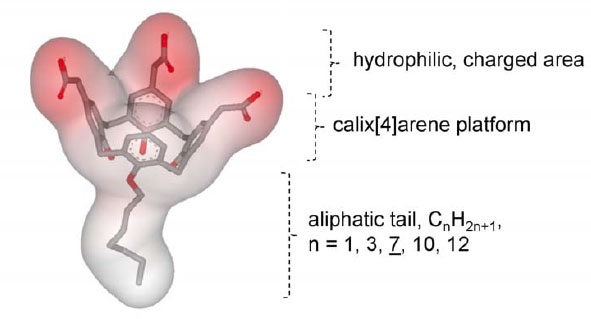
Figure: Basic Structure of Calixarene. Image source: Matar-Merheb R, Rhimi M, Leydier A, Huche´ F, Galia´n C, et al. (2011) Structuring Detergents for Extracting and Stabilizing Functional Membrane Proteins. PLoS ONE 6(3): e18036. doi:10.1371/journal.pone.0018036
Calixarene molecules act as a surfactant in buffers as they form a micelle-like structure of 2-5 nm in a pH-sensitive manner (CMC ranging from 7.5mM-12.5mM).
Calixarenes enhance protein stability by two mechanisms:
- Formation of salt bridge network: This is achieved by the interaction of surface reactive groups of the extracted proteins with the Calixarene molecules resulting into supramolecular clusters. The clusters facilitate the strengthening of the domain-domain interaction of the protein and allow preservation of the native state of the protein.
- Charge shielding: Calixarene molecules form a cage-like structure around the lysines present on the surface of the extracted protein, consequently shielding the overall charge. This, in turn, modulates the electrostatic surface properties of the protein, facilitating better packing and purification of the extracted protein.
Calixarenes allow a wide window of purification and step-up modifications by stabilizing the protein/lipid/detergent complex. Detergents can easily be removed afterward.
Several functioning membrane proteins have been purified with the new Calixarenes:






