Routine H&E (Haemotoxylin and Eosin stains) and special staining comes especially handy when examining tissue structure and cell types and/or when looking for the presence of certain microorganisms in a sample. While both stains are used in histopathology laboratories, H&E stain is more commonly used by pathologists and researchers for investigating underlying cellular and tissue structures.
The Protein Man

Recent Posts
Key Differences of H&E and Special Stains for Immunohistochemistry
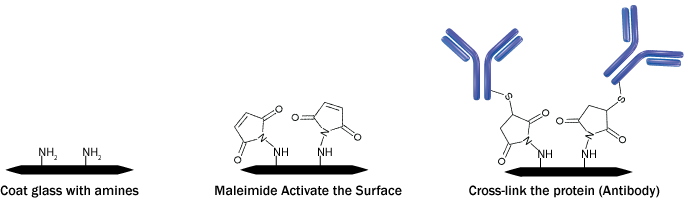
Study Protein to Protein Interaction with Protein Cross Linking to Glass
In all the complex biological processes, the mechanism underlies a synchronized and orchestrated molecular organization, which works through protein-protein interactions. These interactions could be transient, such as catalytic or signal-transduction pathways, requiring a transitory protein-protein association. Alternatively, stable or semi-stable multi-protein complexes are also formed in many biological functions. Hence, in order to identify transient and semi-stable protein associations, chemical cross-linking of proteins came as a useful technique. It involves the formation of chemical covalent bonds between the interacting protein utilizing bifunctional reagents consisting of reactive end groups, which can react with the functional groups of amino acid residues, such as primary amines and sulfhydryls. The formation of cross-links provides direct and concrete information regarding the identity of the interacting proteins as well as the regions of contact between the proteins.
Topics: Cross-Linkers
When to Use Immunohistochemistry (IHC) Detection vs. a Direct Method
How do you know which method to use for visualizing proteins after immunodetection? Should you be using the direct detection method or would applying an indirect detection method best serve your purpose? If you’re not sure which method to use, here are some things you definitely need to know.
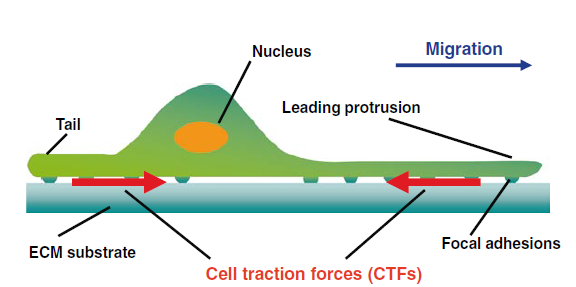
Traction Force Microscopy for cell interaction with extracellular matrix
The fundamental source of all processes in living system is force. Force is required to maintain the balance between the external and internal environment of any living system. Cells have the ability to sense and respond to the forces. Cells perform different activities such as migration and interaction with extracellular ligand. While performing all these vital functions required of normal development and maintenance of homeostasis, cells perform a myriad of feedback mechanisms in order to adjust with the mechanics to correlate with the physiological needs of the cell. Hence, in a nutshell, cells have a dynamic reciprocation of exertion and resistance force to tune with the material properties of the extracellular matrix and help to assert physical interactive forces with the neighboring cells or with the extracellular milieu. Consequently, it is essential to measure these cellular forces to understand different functions and properties of the cell. Cellular forces are not evenly distributed in space and vary according to the substrate. When cells are cultivated on a specific substrate, especially when grown in-vitro, the forces, also known as cell traction forces, are exerted by the cells at localized regions known as focal adhesion sites which is subsequently attributed to the physical properties of the cell and the substrate interaction.
Topics: Teaching Biotechnology