How do you know which method to use for visualizing proteins after immunodetection? Should you be using the direct detection method or would applying an indirect detection method best serve your purpose? If you’re not sure which method to use, here are some things you definitely need to know.
Direct Detection Method: The Pros and Cons
The direct detection method has been gaining popularity, especially among researchers doing immunofluorescence and flow cytometry studies, for a number of reasons. For one, direct detection eliminates the need for an additional incubation step and a secondary reagent. The primary antibody can be directly labeled and conjugated to an appropriate enzyme (e.g., horseradish peroxidase (HRP), alkaline phosphatase (AP)) or fluorochrome. In addition, the availability of a wide range of fluorochromes makes designing multicolor experiments a breeze.
Despite the apparent advantages, there are some drawbacks in using the direct detection method:
- It is not as sensitive as the indirect detection methods. The direct detection method is ideal for applications where there is an abundance of target proteins and the primary antibody is highly specific.
- The primary antibody is tagged with a label, making it unsuitable for other applications down the line.
- Considering the fact that there is a limited range of directly labeled primary antibodies available, you may need to label your own antibodies.
Please note that this method of protein detection is recommended when you are trying to detect highly expressed antigens. For other applications, especially those involving poorly expressed antigens, you may need to consider using indirect IHC methods.
The Beauty of Indirect Detection Methods
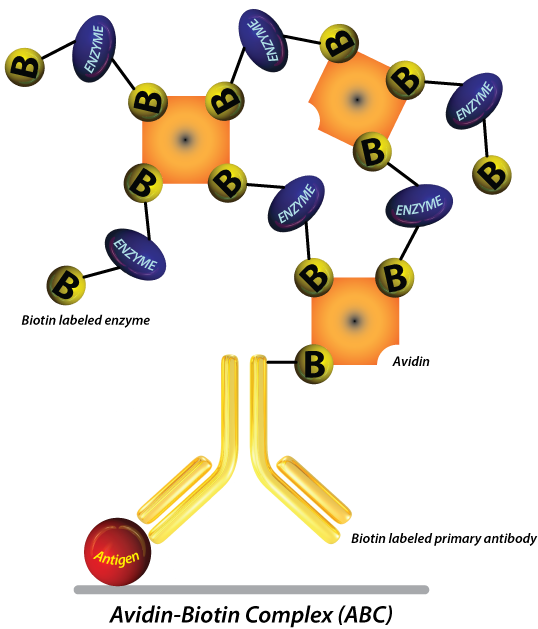
While the direct method relies on the use of a directly tagged antibody for detection, indirect IHC detection methods make use of an unconjugated primary antibody and a labeled secondary antibody. The signal is usually amplified even further by the use of biotinylated secondary antibodies and avidin or streptavidin coupled enzymes (HRP or AP). This is often referred to as “ABC” (Avidin Biotin Complex) detection. Polymer based technology that works in a similar manner is also commercially available. Here are some reasons why most researchers choose these methods to detect their protein of interest.
- It offers greater sensitivity. The presence of a secondary antibody amplifies the signal and increases the sensitivity of the assay, so you don’t have anything to worry about – even if your target protein molecules are low in abundance or are poorly expressed. With two or more secondary antibodies binding to the primary antibody, the signal produced by poorly expressed antigens is significantly improved.
- The use of biotinylated secondary antibodies allows for great flexibility since the same ABC kit could be used to detect all of your antibodies while amplifying sensitivity even further.
- It is more flexible. There is a wide range of conjugated secondary antibodies and detection labels (e.g., light emitting fluorescent labels and chromogenic enzymes) in the market so you can detect your primary antibody in more ways than one!
Note: This method requires additional blocking steps to reduce the risk of non-specific binding and high background staining.
Indirect Detection: Which Type Should You Use?
There are several types of indirect detection methods used in IHC. The most popular and perhaps most established one is probably the ABC method. However, polymer based detection kits that function in a similar manner are becoming increasingly popular. Kits that are capable of detecting several species of primary antibody while amplifying signal are particularly versatile.
Chromogenic detection has the advantage of being exceptionally stable and not being susceptible to photo-bleaching. When performed in paraffin embedded tissue sections the slides are very durable and can be analyzed by several people without having to take pictures right away or worry about photo-bleaching. Signal amplification methods such as the ABC more than overcompensate for any advantage in sensitivity that a directly labeled fluorescent secondary antibody would give. This is usually the method of choice for analyzing paraffin embedded tissue sections.
Choosing between a fluorescent or a chromogenic detection method might be more of a personal preference. Fluorescent detection has the advantage of having the potential to amplify the signal and sometimes being easier to digitally quantify signal. This method offers the advantage of easier multiplexing, better co-localization of target and higher dynamic range. It also requires no additional step for substrate addition. It is usually more popular with frozen sections or IF studies in research settings.
Related Blogs






