Traditional Immunoprecipitation procedure can be divided into four key steps (Figure 1):
- Antigen labeling (Optional)
- Cell lysis to release the antigen
- Formation of antibody-antigen complex
- Capture & purify antibody-antigen complex
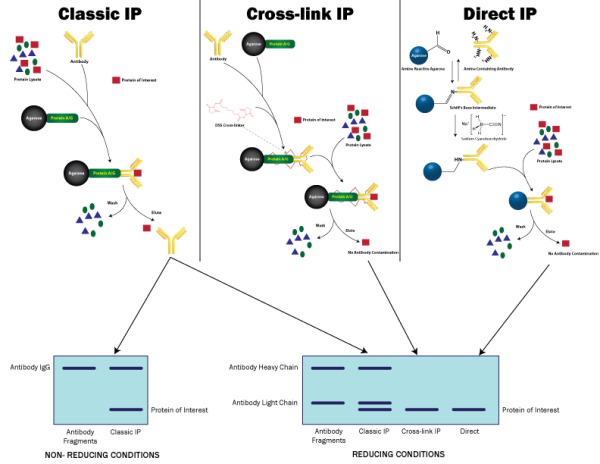
The basic principle involves either the immobilization of the antibody against an antigen of choice to an insoluble solid support, such as an agarose bead. The pre-immobilized antibody is then incubated with a complex protein solution, such as a cell lysate. During incubation with the lysate, the antibody binds the antigen of interest and then the immobilized immune complexes are separated from the lysate.
Alternatively, the free, non-immobilized antibody is incubated direct with the cell lysate and forms immune complexes. These immune complexes are then captured and purified by the addition of an insoluble support that binds to the antibody. This second approach is preferred for samples containing low protein concentrations or the antibody has low affinity for the protein.
Antibody Immobilization
There are two common methods for immobilizing antibodies to the insoluble, solid supports. The first is to use an antibody binding protein that is covalently coupled to the agarose; the second is to covalently couple the antibody directly to the supports.
Protein A, Protein G & Protein A/G Binding Proteins
Protein A. Protein G and Protein A/G are antibody (immunoglobulin G (IgG)) binding proteins that bind the constant domain of antibodies. This feature allows for the correct orientation of the antibodies, the antigen binding domain is orientated away from the support.
Protein A, Protein G and Protein A/G have different affinities for antibodies from different species, as well as different subclasses of immunoglobulin Gs. Protein A/G is a recombinant protein consisting of the key antibody binding domains of both Protein A and Protein G. It is in fact made up of 4 Protein A and 2 Protein G antibody binding domains.
In some cases the use of the Protein A, G and A/G IgG binding proteins in immunoprecipitations is not suitable due to the selection of antibody used. A prime example would be the use of Chicken IgY antibodies.
An inherent problem of using Protein A. G and A/G in immunoprecipitations is that the antibody is not covalently bound to the antibody binding proteins. This results in the release of the antibody and antigen complex during elution and in some cases the heavy (55kDa) and light (25kDa) chains of the antibody may interfere in detection of the protein antigen of interest.

Cross-linking
Protein cross-linking can be used to overcome the issue of the antibody fragments eluting with the protein of interest when using Protein A, G or A/G. The use of small, amine reactive homobifunctional protein cross-linkers, such as DSS and BS3, will covalently attach the antibody to the Protein A, Protein G and Protein A/G antibody binding proteins. The antibody would first be coupled to the Protein A, G or A/G agarose beads and then incubated with an appropriate cross-linker. The antibody would then be covalently coupled to the support and ready for use in the immunoprecipitations.

Direct Covalent Immobilization
The use of direct covalent immobilization binds the antibody directly to the insoluble, solid support therefore eliminating the requirement for antibody binding proteins, such as Protein A, G or A/G. In addition, because the antibody is covalently coupled to the beads there will be no co-elution of the antibody with the antigen, as seen with classical immunoprecipitations using antibody binding proteins.
There are several commercially available products that bind amine residues to an agarose support and these are utilized for the direct, covalent binding of antibodies. This method couples antibodies in a random orientation, however the effect of antibody:antigen interactions and IP capacity is slight. In addition, the advantage of not having the antibody co-elute makes this technique highly popular.

Summary
- Classical: Highest capacity, some restrictions in antibody types used, antibody eluted with antigen
- Crosslinking: Siginificant loss in binding capacity, some restrictions in antibody types used, antibody retained on resin
- Direct: Some loss in binding capacity, suitable for all antibodies, antibody retained on the resin