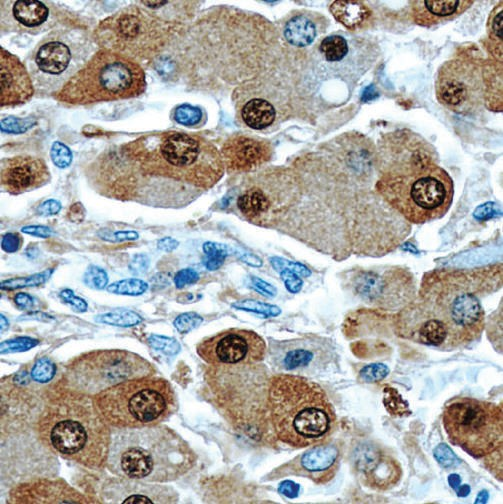
Immunocytochemistry is one of the most commonly used methods for protein localization in the cells or tissues using specific antibodies. The overall credibility of the results from Immunocytochemistry depends on two factors:
Specificity of Immunostaining methods: How to choose a control?
Topics: Protein Labeling, Teaching Biotechnology, Cytology & Histology
Sample preparation for Mass spectrometric analysis
Mass spectrometric analysis is one of the important technique in protein biochemistry and peptide analysis. It is used to identify and analyze proteins and peptide samples. Care should be taken for sample preparation for mass spec analysis as the quality of data depends on the purity of sample applied. A variety of strategies are used to prepare the samples for mass spectrometric analysis.
Blue Native Polyacrylamide Gel Electrophoresis: A simple tool for characterization of Protein Complexes
Proteomics is a complex field and it is exposed to major developments over the last few decades. A general proteomic experiment is a multi-step process, which involves modifications and analysis at every step depending on the experimental requirement. Crudely, these experiments have the following steps:
Topics: Protein Electrophoresis
Strategies to stabilize aggregate-prone proteins in E.coli
Maintaining protein function and stability during the entire process of protein expression and purification is crucial. Though a lot of strategies are being implemented by many researchers, each protein has its own requirements for its stability. Some proteins are prone to aggregation, a small trigger is enough to destabilise the protein at any stage of purification. Each and every step of protein purification is challenging for maintaining stability of aggregate-prone proteins. Few strategies to be considered while purifying these sensitive proteins starting from cloning to storage of pure protein.
Topics: Protein Extraction