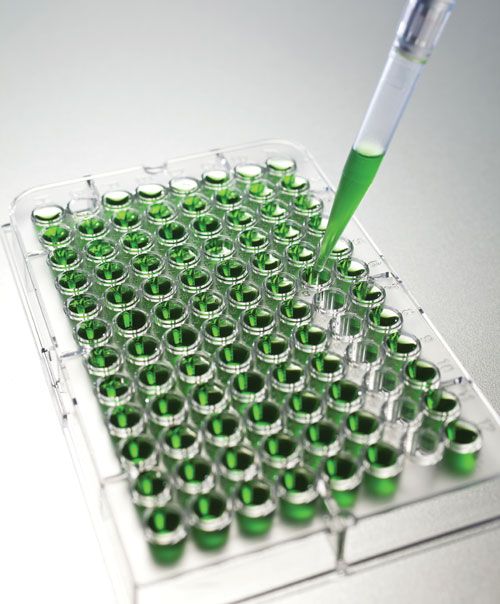
When it comes to detecting and quantifying the concentration of an antigen in an unknown sample, most researchers depend on ELISA (Enzyme-Linked Immunosorbent Assay). This is completely understandable since this plate-based assay technique has a completely robust nature, exhibits exceptional sensitivity and is quick and easy to perform.
The Most Basic Biotech Lab Equipment, Materials & Necessities
G-Biosciences and Ellyn Daugherty have compiled a simple list of equipment and materials required for a new biotechnology teaching lab. When setting up a new lab, it is often quite daunting, as it is hard to budget and plan if you are unsure of the basic requirements for the lab. This blog is designed to highlight the key requirements for a lab to accommodate 8 lab groups.
Topics: Teaching Biotechnology
Physical Disruption or Chemical Cell Lysis? How to Decide
Cell lysis is an essential step in the extraction and purification of intracellular proteins, and the method that you use to disrupt your cell samples can determine the success and reproducibility of your downstream applications to a great extent. Keep in mind that one vital component of the starting biological material is inevitably lost in every step of the experiment, so it’s important to get everything you need right from the start. This underscores the need of choosing the cell disruption method that will allow you to extract the best possible yield and purity of your biological materials.
Topics: Protein Extraction
Protein extraction from plant, animal, fungi, bacteria, etc is well known using different techniques. Many buffers are used to solubilize water-soluble proteins. There are few proteins which are insoluble and are rigid to be extracted by physical means or they lose activity when extracted by denaturants. One of the promising methods is to use organic solvents to extract proteins inactive state. C4 and C5 alcohols (butanol, pentanol) are mostly used for the purpose as they act as differentiating solvents. When an organic solvent is added to a protein solution followed by ammonium sulfate, the solution separates into three phases. Upper organic phase and lower aqueous phase, depending on the concentration of ammonium sulfate some proteins precipitate out and form an intermediate layer.