Proteomics is a complex field and it is exposed to major developments over the last few decades. A general proteomic experiment is a multi-step process, which involves modifications and analysis at every step depending on the experimental requirement. Crudely, these experiments have the following steps:
- Biochemical Fractionation of the sample for separating proteins based on their properties
- Enzyme-based protein digestion into smaller peptides
- Liquid chromatography-mass spectrometry (liquid chromatography combined with tandem mass spectrometry or LC-MS/MS) for peptide-mixture analysis
- Database-dependent data analysis and interpretation
Although, each and every step of the proteomic analysis is crucial the purity analysis and biochemical fractionation of the sample holds a pivotal position. Majority of the sample fractionations are detergent based using sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which separates proteins based on their molecular weight under denaturing and reducing conditions. However, protein fractionation in native conditions is yet another method which can keep the activity of the proteins intact before LC-MS/MS analysis and can generate a plethora of information. Of all the available methods in native fractionation, Native PAGE (BN-PAGE) and Colorless Native PAGE (CN-PAGE) are most preferred.
Blue native polyacrylamide gel electrophoresis is a very common method implicated for characterization of proteins in their enzymatically active state with a high-resolution separation. In this method, a dye, Coomassie Blue-G250, is used for visualization and induction of external negative charge on the protein complexes. The complexes are then separated based on their molecular weights contrary to that of conventional SDS-PAGE in which the proteins are fractionated based on their charge/mass ratio. In BN-PAGE, the protein complexes tend to migrate according to the pore size of the gradient gel till they reach the pore size limit point.
Coomassie Blue-G250 dye provides many advantages over SDS such as:
- It is anionic in nature; hence allow coating of the complexes with negative charge without denaturation.
- The solubility is higher in water
- It has a unique capability of binding with membrane proteins, therefore the best tool for membrane proteins fractionation. There is a minimal probability of aggregation of membrane proteins because of the charge distribution. Also, the interaction of the dye with the membrane proteins facilitates the loss of their hydrophobicity, eventually making them water-soluble.
However, other things are important for better results such as low concentration of mild detergents such as digitonin or dodecylmaltoside and the type of sample for extracting proteins.
BN-PAGE uses gradient polyacrylamide gel for separation. Coomassie blue G-250 is added to the lysate obtained after the solubilization and centrifugation of the sample. During the process of separation, the native proteins and complexes migrate as blue dots or bands through the gradient gel. These bands can be excised to recover the proteins of interest using electroelution method.
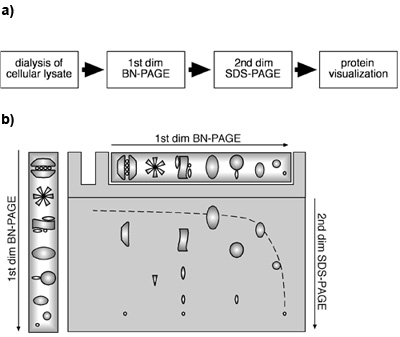
Fig: Schematic representation of analysis of proteins using 1D BN-PAGE and 2D BN-PAGE. (Source: Fiala GJ et al., Journal of Visualized Experiments, 2011)
BN-PAGE is a simple method holding a great power of result and analysis. It provides a considerable amount of information when coupled with other methods such as LCMS or iTRAQ. It also provides details about the size, subunit composition of the protein complexes, stoichiometry, number and approximate abundance of the complexes/proteins in the sample. If compared with SDS-PAGE in the same gel in the second dimension, BN-PAGE can give precious revelation about the interacting partners of a specific protein as well as its probable subunit composition.
Advantages of BN-PAGE:
- Transient interactions can also be studied as the conditions are non-denaturing.
- Two-dimensional BN-PAGE provides subunit composition information.
- It is an exquisite tool for studying comparative protein associations after experimental conditions
- BN-PAGE confirms the results of immunoprecipitation.
Applications of Blue Native Gel Electrophoresis:
- Isolation and fractionation of membrane proteins.
- Fractionation and identification of mitochondrial proteins without aggregation.
- One-step estimation of the native mass of the complex and its polymeric or oligomeric state.
- It allows stoichiometry determination of a protein complex using an antibody-based gel-shift method.
- BN-PAGE is a handy technique for 2D crystallization, electron microscopy, in-gel activity assays, native electroblotting, and immunodetection.
Limitations of BN-PAGE:
- It requires good quality and robust antibodies for detection of the protein. Not all antibodies that are helpful in SDS-PAGE can provide satisfying results with BN-PAGE.
- The resolution is low between protein complexes which require optimization of the gradient gel every time.
- Coomassie dye has shown to interact with few protein-protein complexes resulting in a smear formation on the gel. In such cases, CN-PAGE is preferred over BN-PAGE.






