Protein extraction from plant, animal, fungi, bacteria, etc is well known using different techniques. Many buffers are used to solubilize water-soluble proteins. There are few proteins which are insoluble and are rigid to be extracted by physical means or they lose activity when extracted by denaturants. One of the promising methods is to use organic solvents to extract proteins inactive state. C4 and C5 alcohols (butanol, pentanol) are mostly used for the purpose as they act as differentiating solvents. When an organic solvent is added to a protein solution followed by ammonium sulfate, the solution separates into three phases. Upper organic phase and lower aqueous phase, depending on the concentration of ammonium sulfate some proteins precipitate out and form an intermediate layer.
This method can be easily scaled up from milliliters to liters. Lipids, detergents, few proteins, phenolic compounds, and some low molecular weight contaminants can be separated from proteins by using this method. Few proteins like superoxide dismutases, catalase can easily be isolated from human erythrocytes leaving the major contaminant Hemoglobin in the inorganic phase (t- butanol). Navin et al., successfully extracted proteins with nutritive value using organic solvents from peanuts. Therapeutic proteins like Interferon beta is extracted with 2-butanol. Recently Gagaoua et al purified Zingibain, a milk-clotting enzyme by using t-butanol. There is no effect on the activity of these proteins after organic solvent extraction.
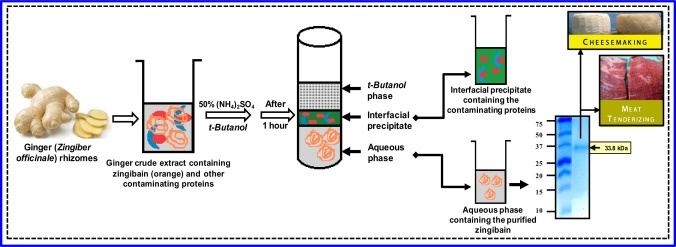
Figure adapted from M. Gagaoua et al. / Data in Brief 6 (2016) 634–639
The following points to be noted while optimizing the purification procedure using organic solvents:
- Estimation of total protein concentration and relative concentration of the desired protein.
- Calibration of bioassay for hormonal or enzyme activity
- Optimization of ammonium sulfate concentration, usually half the amount used for normal salting out is enough
- Adjusting the pH using different buffering solutions ( between pH 3-7). Preferably 2 to 4 pH units less than the isoelectric point of the desired protein
- Optimization of temperature for sensitive proteins is important. Some proteins can be purified at room temperature, whereas some require low temperatures
- All three phases should be checked for the desired protein as some proteins go to the organic phase, some to the aqueous phase and some in the intermediate layer.
An example protocol for purification of proteins using t-butanol:
- Starting material (cell paste or tissue or plant material) is first homogenized and sonicated.
- The lysate is clarified by filtration or centrifugation.
- The clarified lysate is dialyzed into the desired buffer and 50 % of ammonium sulfate is added.
- Then t-butanol is added in 1:1 ratio and incubated for 1hr at room temperature followed by centrifugation at 15,000rpm for 10-15 minutes.
- All three layers are checked on SDS-PAGE along with crude extract.
- If the desired protein is in the aqueous phase, it is dialyzed overnight against suitable buffer and stored at -80 ℃ till further use. If the protein is precipitated it can be redissolved in a suitable buffer and stored.
References:
- Navin Neucere and Robert L. Ory, Effect of organic solvents on the proteins extracted from peanuts. Journal of Agricultural and Food Chemistry 1968 16 (2), 364-365
- Mohammed Gagaoua, Kahina Hafid, Naouel Hoggas, Data in support of three phase partitioning of zingibain, a milk-clotting enzyme from Zingiber officinale Roscoe rhizomes, Data in Brief, Volume 6,2016,Pages 634-639,ISSN 2352-3409, https://doi.org/10.1016/j.dib.2016.01.014






