The stability of proteins is crucial in many in vitro protein studies and is considered a major requirement in functional studies involving native and recombinant proteins. Thus, understanding protein stability and preserving the native conformation and normal functions of your protein of interest can be very helpful when working with your protein of interest.
The Protein Man

Recent Posts
Topics: Protein Purification, Protein Extraction
The Advantages of Using Trypsin for Mass Spectrometry
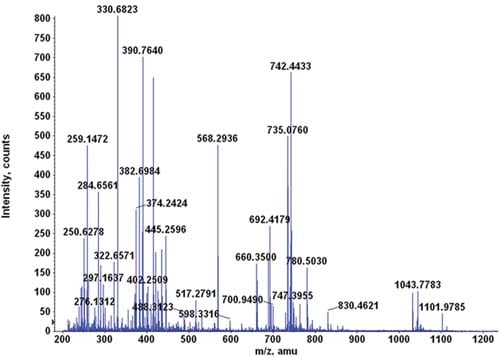
In mass spectrometry-based proteomics, nothing comes close to trypsin in breaking down protein mixtures into peptide fragments. In fact, protein researchers consider trypsin as the runaway winner when it comes to protease activity and specificity – and there are a lot of good reasons why they do.
Topics: Mass Spectrometry
When it comes to labeling your antibodies for downstream application requiring signal detection, you have two choices. You can either go for the direct approach or the indirect approach. How do you know which method to use? Here are some things you need to know to make sure you pick the right method for the intended application.
Topics: Antibody Production, Protein Labeling
Expression of recombinant proteins with peptide or protein tags is widely used in protein research for three main reasons, ease of purification from a large pool of host proteins, enhancing solubility of the protein and for localization studies. Some important steps to be considered while choosing an expression vector are compatibility of tag sequence with that of the desired protein, codon usage, including linker sequences, peptide cleavage sites and the impact of the tag on the nature of desired protein. Various tags are used ranging from large proteins (Maltose Binding Protein {MBP}) to small peptides (Hexa (6X) Histidine). Tags can be added to either N-terminal side or C-terminal side of the desired protein.
Topics: Protein Purification, Molecular Biology

.jpg?width=788&name=Protein%20Structure%20(8).jpg)