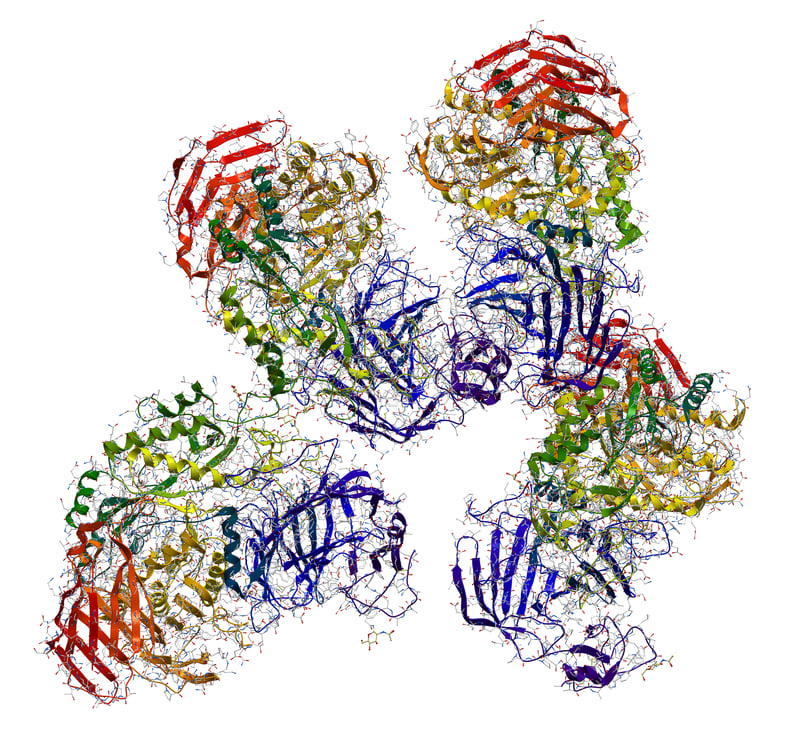
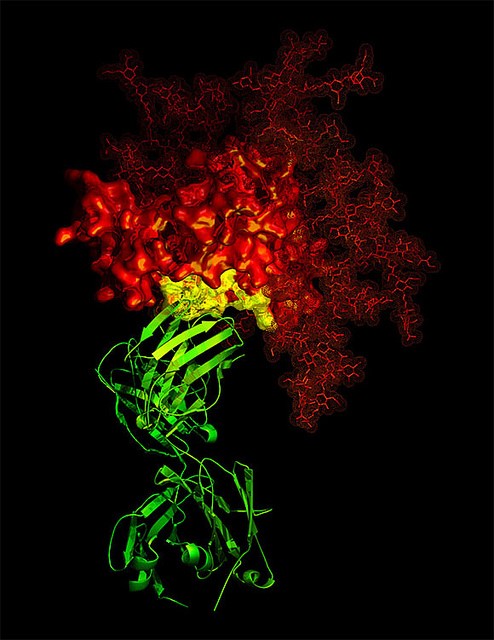
Host cell proteins (HCPs) are one of the main impurities in bio-therapeutics that are carried over from the host expression system. Although HCPs are at low levels, they can trigger immunogenic reactions in the human system when administered along with bio-therapeutics. The similarity of physicochemical properties of HCPs and therapeutic proteins result in co-purification. Detection and elimination of these HCPs during bio-therapeutics purification are required. Development of analytical tools and methods for the detection of these HCPs during protein purification and quality control is a challenge. The final threshold level of HCPs varies for different bio-therapeutics, so manufacturers have
to analyze and report the HCP levels at all stages of downstream processing before the
product is released according to ICH-Q6B guidelines.
Host Cell Protein Analysis: Detection in Biotherapeutics
Topics: Protein Detection
Best Blocking Buffer Selection for ELISA & Western Blot
Antibodies are used to detect specific proteins during research. There are a variety of tests including the Western blot, that produce a surface area that can contain other proteins and components that need to be blocked. Blocking buffers are used to bind the surface areas that may attach to reagents during the testing process, preventing the non-specific antibodies from interacting with the reagents and causing a poor reading or inaccurate results. Knowing which buffers to use, however, is critical for ensuring the accuracy of a test, as each option has its own advantages and disadvantages.
Topics: Western Blotting, Assay Development (ELISA), Protein Detection
How to visualize proteins after electrophoresis
How to visualize proteins after electrophoresis
In-gel visualization of proteins separated by SDS-PAGE or by 2-D gel electrophoresis is achieved by staining gels with dye, metals ions or fluorescent stains. Different staining method are preferred depending upon broad range binding of stain to proteins, the sensitivity to detect low abundant proteins and downstream application desired for a separated protein like mass spectrometry. Sometimes two staining methods are carried out as single staining method cannot achieve everything desired by a researcher. Various staining method for in-gel visualization of proteins are as follows.Coomassie Brilliant Blue staining
The Coomassie dyes R-250 and G-250 bind to proteins stoichiometrically through their sulfonic acid groups. The interactions between dye and protein are Van der Waals and ionic. The sulfonic acid groups interact with positive amine groups. Therefore coomassie dye binds to wide range of proteins. The most commonly used dye R-250 can detect protein as low as 0.1 µg. Coomassie Brilliant Blue involves staining and destaining step as the gel also turns blue. Coomassie Brilliant blue is compatible with mass spectrometry analysis. Though widely used for staining gels to view proteins, its not a method of choice if higher sensitivity is desired. For analytical purposes other methods like silver staining and fluorescent staining are preferred over classical Coomassie Brilliant Blue staining.Colloidal Coomassie G-250 staining
In colloidal Coomassie staining G-250 dye is used instead of R-250 due to its colloidal properties. G-250 forms colloidal particles in high alcoholic solutions containing strong acids and high concentration of salts 1, 2. Formation of colloidal G-250 particles reduces free dye in solution and thus the dye does not penetrate and stain gel. This step enhances the sensitivity of staining proteins and removes background staining of gels. With colloidal Coomassie staining detection limit of 1-10 ng of protein in gel is achieved 3.Silver staining
It offers high sensitivity of less than 1 ng of protein 4. The silver ions bind to the protein and are reduced further with a developing solution to give an image of protein bands. Silver staining involves various steps like protein fixation, sensitization, silver impregnation and image development. Based on solution used there are two methods of silver staining: silver nitrate and silver-ammonia complex method. Silver staining protocols compatible with downstream application like mass spectrometry are available. The downside of silver staining is that it has limited dynamic range and thus do not interact uniformly with all proteins. In addition it involves use of hazardous chemicals.Fluorescent staining
Fluorescent staining uniformly stains protein, is rapid and offers sensitivity equal to that attained by silver staining method. It involves no destaining step and is compatible with mass spectrometry and microsequencing. Commercially fluorescent stains are available to stain 1D or 2D gels to visualize bands.Stain free detection of proteins in gel
In stain free method for visualizing proteins, 2, 2, 2-Trichloroethnaol is added to the polyacrylamide solution before casting a gel 5. The principle behind stain free method is that the tryptophan amino acids in protein undergo an ultraviolet light-induced reaction with trihalocompounds to produce fluorescence in visible range (300 nm). This method is fast as one can see bands on transilluminator within 5 minutes and it avoids use of stains.References
1. Neuhoff, V. et al (1988). Electrophoresis 9, 255-2622. Candiano, G. et al (2004). Electrophoresis 25, 1327-1333
3. Kang, D. et al (2002). Bull. Korean Chem. Soc. 11, 1511-1512
4. Chevallet, M. et al (2006). Nat Protoc.1(4): 1852-1858
5. Ladner, C. L. et al (2004). Anal Biochem. 2004 Mar 1, 326(1):13-20
Topics: Protein Detection
Selecting Protein Assays? These Are the Top Factors That Can Impact Accuracy
The ability to accurately quantify protein concentration is the key to a successful laboratory workflow, and is often required prior to processing protein samples for isolation, separation, and analysis. To determine the total protein concentration in a sample, one of the first factors to consider is the selection of an appropriate protein assay method. However, since there are a wide variety of protein assays available, you need to take a number of factors (e.g. the accuracy required and the amount and purity of the protein available) into account to make sure that you are using the most suitable assay for your application.
Topics: Protein Estimation, Protein Detection