INTRODUCTION:
Many protein modification reagents for biotinylation and cross-linking involve reactive groups that conjugate through primary amines. Primary amines are found in all proteins and peptides as they make up the N-terminus and are also a component of the lysine residue side chains. Amines, lysine ε-amines and N-terminal α-amines, are the most abundant group in protein molecules and represent the most common target for biotinylation. For example, BSA contains 59 primary amines, of which up to 35 are available on the surface of the molecules and can be reacted with amine reactive esters.
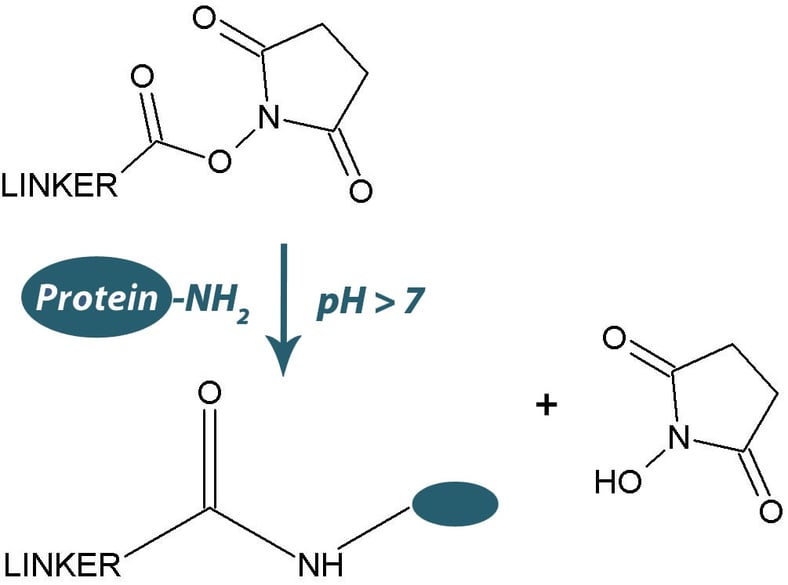
The most widely used amine reactive biotinylation and cross-linking reagents are the water insoluble N-hydroxysuccinimide (NHS) esters or the water soluble N-hydroxysulfosuccinimide (sulfo-NHS) esters.
The addition of a charged sulfonate (SO3-) on the N-hydroxysuccinimide ring of the sulfo-NHS esters results in their solubility in water (~10mM), but are not permeable to plasma membranes. The solubility and impermeability to plasma membranes makes them ideal for studying cell surface proteins as they will only react with the protein molecules on the outer surface of plasma membranes.
Both forms of the NHS esters hydrolyze rapidly in aqueous solutions (7 hours at pH7, minutes at pH9) and for this reason the NHS esters must be handled and stored appropriately. The NHS esters should be stored desiccated and by allowed to warm to ambient temperature before opening to avoid condensation. That being said, repeated opening and closing and inappropriate handling will lead to the introduction of moisture and hydrolysis of the NHS-esters.
Hydrolysis (and conjugation) results in the release of NHS that can be assayed with the following procedure.