 There are several contemporary methods for protein purification. Some techniques are very powerful, such as reverse phase chromatography, immunoadsorption, or affinity chromatography. However, these methods may ultimately denature protein and negatively impact the desired product. Likewise, recombinant proteins recovered from inclusion bodies are often aggregated and nonfunctional. In all of these situations, the purified protein product must be renatured for further use.
There are several contemporary methods for protein purification. Some techniques are very powerful, such as reverse phase chromatography, immunoadsorption, or affinity chromatography. However, these methods may ultimately denature protein and negatively impact the desired product. Likewise, recombinant proteins recovered from inclusion bodies are often aggregated and nonfunctional. In all of these situations, the purified protein product must be renatured for further use.
Refolding of proteins requires transitioning from a denatured conformation to a folded, functional structure. During this transition, the protein proceeds through one or multiple intermediates. Problems can arise during this process, because the proteins typically have exposed hydrophobic regions that tend to aggregate together and inhibit any further folding. An ideal folding environment will inhibit and therefore slow down aggregation while not interfering with the correct folding path. The folding path of every protein is different, but there are general guidelines that can be followed to find and optimize the correct conditions to allow protein renaturation:
- The aggregation reaction involves several protein molecules so reducing the protein concentration should make it less likely for them to clump together to form an aggregate.
- Reducing temperature will reduce the rate of aggregation.
- Use denaturants such as urea, guanidine, arginine, polyethylene glycol, or detergents at a high enough concentration to inhibit aggregation but low enough to allow the protein to still fold somewhat.
It should also be taken into consideration that these factors affect both the aggregation and correct folding reactions. Because of this, Non-detergent sulfobetaines (NDSB’s) are the preferred agents for denaturant protein purification. NDSB’s are able to effectively fold proteins without denaturing the product. Many traditional protein solubilization compounds, such as urea, are not able to accomplish this task without negatively impacting proteomic quality.
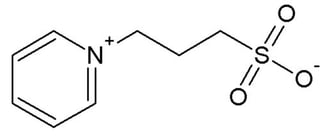
Non-detergent sulfobetaines are zwitterionic compounds that have a hydrophilic sulfobetaine head group and are similar to other zwitterionic detergents with the exception that NDSB’s are much shorter in length than typical detergents. This shortness makes it very unlikely that NDSB’s will form micelles even at higher concentrations of 1M. This unique feature allows NDSB’s to solubilize proteins without denaturing them and also makes it much easier to remove NDSB’s from solution by dialysis. Furthermore, NDSB’s do not absorb UV light significantly at 280 nm so they will minimally interfere with UV protein quantification, and are also zwitterionic over a wide pH range. Each of these aspects makes NDSB’s ideal candidates for usage in protein purification.
There are a variety of NDSB compounds, some of which serve better in certain circumstances. In general, NDSB’s with larger hydrophobic tails yield better results. NDSB’s with aromatic rings typically are very effective agents, due to the effects of aromatic stacking on denatured proteins. Compounds with cyclic structures tend to perform better than chains.
Unique situations may affect which type of NDSB agents are most effective on different protein compositions. The best protocol will vary, and each protein purification process should consider the unique characteristics of the protein and desired product when choosing the best solubilization agent. Overall, NDSB’s are versatile and effective, and are a great addition to many protein purification protocols.
Related Blog Posts






