 Extraction of proteins from cells and tissue of organisms is the first step towards isolation of proteins. The extracellular matrix needs to be removed or digested in case of tissue, the cell wall needs to be digested for organisms like bacteria, yeast and plants, and the cell membrane needs to be disrupted to release the proteins in solution. Traditionally, physical methods for disruption of cells and tissues are employed to release cellular proteins including sonication, french press, homogenization, manual grinding or using blenders. Although one is able to get the active proteins, these physical methods have several limitations:
Extraction of proteins from cells and tissue of organisms is the first step towards isolation of proteins. The extracellular matrix needs to be removed or digested in case of tissue, the cell wall needs to be digested for organisms like bacteria, yeast and plants, and the cell membrane needs to be disrupted to release the proteins in solution. Traditionally, physical methods for disruption of cells and tissues are employed to release cellular proteins including sonication, french press, homogenization, manual grinding or using blenders. Although one is able to get the active proteins, these physical methods have several limitations:
- Expensive
- Cumbersome due to use of heavy equipment
- Reduced yield as the sample is processed through several steps
- Batch to batch variation in protein yield due variability and handling
Mechanical methods can sometimes denature proteins as these methods are harsh and some of them generate heat and can denature protein if appropriate cooling of sample is not done.
Chemical methods for cell disruption using lysis buffers with ionic detergents can be used to release proteins; however they can denature the protein. Non-ionic detergents offer advantage over lysis buffer and ionic detergents as they are mild and the proteins are not denatured. Therefore, nowadays popular methods for extraction of biologically active proteins from cells or tissues use non-ionic detergents along with other additives, such as enzymes for cell wall disruption and addition of protease inhibitors to inhibit proteases. This method may involve mild mechanical methods, including brief homogenizing or vortexing depending upon the cell or tissue type.
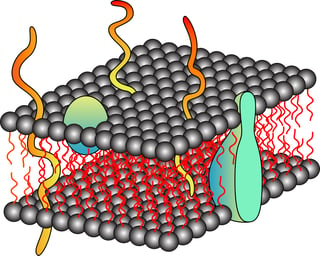
Detergents are organic amphipathic molecules that comprise of hydrophilic headgroup and hydrophobic hydrocarbon moiety. In case of non-ionic detergent the hydrophilic head group is uncharged. Biological cell membranes are lipid bilayers composed of amphipathic phosolipids where the hydrophilic groups are exposed to the cytosol and extracellular matrix and the hydrophobic groups are buried inside to form lipid bilayers. Detergents being amphipathic molecules insert themselves into the cell membranes and disrupt them. The detergents form micelle in aqueous buffers at a particular concentration. The micelle is spherical molecule with their hydrophilic group exposed to aqueous solutions and hydrophobic group buried to form core of the micelle. The hydrophobic cores of micelle bind to the hydrophobic regions of proteins and solubilize them. The ionic detergents have charged hydrophilic group which denature the proteins. Non-ionic detergents break only lipid- lipid and protein-lipid interaction and not protein-protein interactions and thus are predominantly non-denaturants. The non-ionic detergents disrupt the cell membranes to release proteins in solution, solubilize the membrane proteins and does not denature cellular or membrane proteins. There are several non-ionic detergents used for cell lysis like Triton X-100, NP-40 (nonylphenoxypolyethaoxy ethanol), nonidet P-40 (octylphenoxypolyethoxy ethanol) etc. The non-ionic detergent is chosen according to the subcellular location of desired protein, downstream application and its own physical property like Critical Micelle concentration or CMC etc. For example the Triton X-100 has aromatic rings that absorb at 260-280 nm and is thus not recommended if UV monitoring of protein concentration is required for downstream applications. NP40 is not good for extraction of nuclear proteins as its mild detergent and can only disrupt plasma membrane. Thus several different types and concentrations of non-ionic detergent buffers for cell lysis are offered commercially depending upon the location of desired protein and downstream applications. Also in these non-ionic detergent buffers, enzymes are added depending upon which cells are lysed to release proteins. Bacterial cells, yeast cells, fungi and plant cells had additional barrier in form of cell wall which needs to be broken before the non-ionic detergents can interact with cell membranes to disrupt them. Lysozyme breaks bacterial cell wall, zymolyase digest the yeast cell wall, chinitase is used to break fungi cell wall and cellulases are used to break open plant cell walls. In addition protease inhibitors are added to these non-ionic detergent buffers to prevent action of proteases. Though non-ionic detergents does not denature proteins, the excess of it can be easily removed if desired by researcher by hydrophobic adsorption, dialysis or by size exclusion chromatography.








