 ImmunoPrecipitation (IP) is a very useful immunochemical technique for isolating and purifying target proteins out of a complex sample (lysate). In conjunction with western blotting and/or other assay techniques, IPs can be used to determine: the presence and quantity of a protein; molecular weight of a polypeptide; rate of synthesis or degradation; enzymatic activity; and to identify certain post-translational modifications and interactions with other proteins, nucleic acids and ligands. There are different IP strategies to purify and analyze your target protein. Read on to see what IP strategy is perfect for you.
ImmunoPrecipitation (IP) is a very useful immunochemical technique for isolating and purifying target proteins out of a complex sample (lysate). In conjunction with western blotting and/or other assay techniques, IPs can be used to determine: the presence and quantity of a protein; molecular weight of a polypeptide; rate of synthesis or degradation; enzymatic activity; and to identify certain post-translational modifications and interactions with other proteins, nucleic acids and ligands. There are different IP strategies to purify and analyze your target protein. Read on to see what IP strategy is perfect for you.
- Standard Immunoprecipitation
- Co-Immunoprecipitation (Co-IP)
- Chromatin Immunoprecipitation (ChIP)
- RNA Immunoprecipitation (RIP)
- Tagged IP
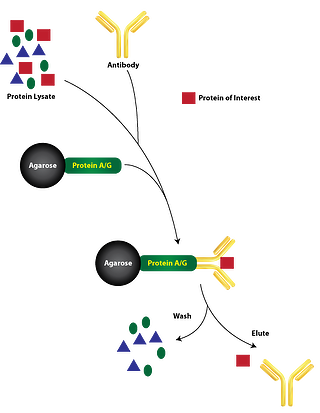
Standard Immunoprecipitation requires the immobilization of an antibody specific to the target protein onto a solid support, such as agarose or magnetic beads. These beads are usually cross-linked with Protein A, Protein G, or Protein A/G (immunoglobulin G binding proteins specific to the species of antibody) which covalently binds the antibodies to the beads. Then the immobilized antibody is incubated with the cell lysate containing the target protein. The target protein binds to the immobilized antibodies forming the immune complex (target protein, protein A/G, and immobilized antibody). The resulting immune complex is precipitated out of the lysate through centrifugation and undergoes several washes to remove nonspecific cellular components. The target protein is then released from the immune complex with an elution buffer that disrupts the antigen-antibody affinity interaction. The target protein can then be eluted and analyzed through other assays.
Direct method is described in the paragraph above, where specific antibodies are immobilized onto a solid substrate such as protein A and/or G agarose or magnetic beads and incubated with the cell lysate. This is the default and preferred method (see figure).
Indirect method is when specific antibodies are added directly to the lysate to bind their target protein. Then the protein A and/or G beads are incubated with the lysate to bind the antibody-protein complexes (see figure). The procedures for both methods converge from this point on. This method is preferred when the target protein concentration is low, when the antibody affinity toward the target protein is weak, or when the binding kinetics of the antibody to the protein is slow.
Co-Immunoprecipitation is used to immunoprecipitate protein complexes (target protein plus bound proteins or ligands) from a cell lysate. This technique is commonly used to examine protein-protein interactions. Much like standard IP, this process requires an antibody specific to the target protein which is believed to be part of a larger complex of proteins. The proper elution buffer must be used to separate the target protein complex from the immune complex. Elution buffers with low ionic strength containing non-ionic detergents such as NP-40 and Triton X-100 are less likely to disrupt protein-protein interactions. The eluted protein complex can then be analyzed through other methods like western blotting and mass spectrometry to identify the interacting proteins. The protein-protein interactions can be validated with a second Co-IP with a different antibody specific to one of the resulting interacting proteins.
Chromatin Immunoprecipitation is used to examine protein-DNA interactions occurring inside the nucleus of live cells or tissues. ChIP can identify regions of the genome that DNA-binding proteins associate, such as histones and transcription factors. ChiP assays require the DNA bound proteins to be temporarily crosslinked and the nucleotide sequences sheared prior to cell lysis. Formaldehyde is commonly applied to the cells or tissue to crosslink the protein-DNA complexes. Following crosslinking, the cells are lysed through sonication and the DNA is fragmented into 0.2-1.0 kb pieces. Then the protein-DNA complex can be immunoprecipitated with an antibody specific to the target DNA binding protein. The formaldehyde cross-linking is reversed by heating the purified protein-DNA complexes which allows the DNA to be released from the target protein. PCR can then be used to identify and quantify the isolated DNA fragments. Alternatively, ChIP-sequencing or microarrays can be used to locate where the protein binds on a genome-wide scale.
RNA Immunoprecipitation is very similar to ChIP, except that antibodies specific to RNA-binding proteins are used to immunoprecipitate protein-RNA complexes. Purified RNAs can then be identified and quantified with RT-PCR and cDNA sequencing.
Tagged IP overcomes the limitation of antibody specificity with other IP approaches. They are dependent on the availability of specific antibodies for a target protein, and therefore, many proteins cannot be immunoprecipitated. To overcome this obstacle, tags are engineered onto the C- or N- terminal of the target protein. This can be very advantageous because the same high-affinity, tag specific antibody can be used. Some common tags for this method are Green Fluorescent Protein (GFP), c-Myc, Glutathione-S-transferase (GST), Hemagglutinin, and the FLAG-tag. It is important to note that although this method is convenient, there are concerns regarding biological relevance because the introduced tag may interfere with native interactions and protein function.
Related Blogs
Advantages of Magnetic Beads for Protein Immunoprecipitation
Protein Immunoprecipitations: Which method to use?






