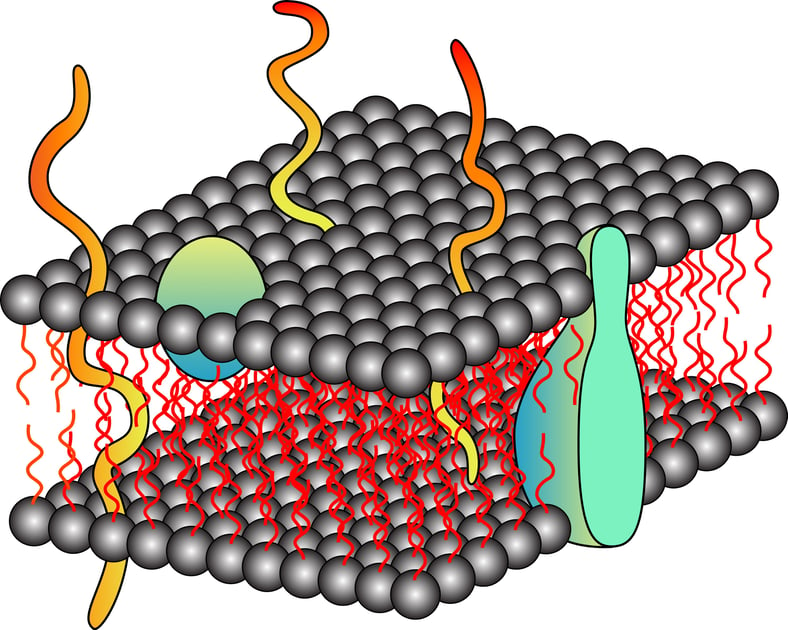
Extraction of proteins from cells and tissue of organisms is the first step towards isolation of proteins. The extracellular matrix needs to be removed or digested in case of tissue, the cell wall needs to be digested for organisms like bacteria, yeast and plants, and the cell membrane needs to be disrupted to release the proteins in solution. Traditionally, physical methods for disruption of cells and tissues are employed to release cellular proteins including sonication, french press, homogenization, manual grinding or using blenders. Although one is able to get the active proteins, these physical methods have several limitations:
The Protein Man

Recent Posts
How to extract biologically active proteins from the cells and tissue of organisms using detergent based lysis method
Topics: Detergents, Protein Extraction
Role of Proteases in Mass Spec Identification of Proteins
Protein analysis and identification through mass spectrometry first requires a breakdown of each protein into their composite peptides. Once the protein has been broken down, the peptides can be separated through the use of a reverse phase column and the peptides and peptide fragments can be measured using a mass spectrometer.
Topics: Mass Spectrometry
Adjuvants play an important role in the antibody production by acting as immunopotentiators. They augment immune response via different mechanisms depending upon the adjuvant such as ‘depot’ effect, antigen presentation, antigen targeting, immune activation or modulation and cell-mediated response. The goal of adjuvant for antibody production is that high affinity, high titer and high avidity (for polyclonal) antibodies are raised. Both humoral and cell mediated response are necessary to achieve efficient antibody production. There are hundreds of preparations for adjuvants described in literature depending upon specific needs of an investigator. Nevertheless, most commonly and widely used adjuvants for antibody productions are few as listed below.
Topics: Antibody Production
How to Check Western Transfer before using expensive antibodies
Immunodetection of proteins on a Western blot requires the use of expensive antibodies, but poor or incomplete transfer would result in wasting these antibodies. A Western transferred blot can be checked for success of protein transfer with a protein stain and therefore prevent wasting expensive antibodies. Various options for protein stains for Western blot are available, including, but not limited to, Ponceau S, Amido black 10B, Coomassie brilliant blue R250, India ink or colloidal gold. In addition to checking success and quality of Western transfer, staining blots is also used for semi-quantitative protein estimation. In addition, checking the blot with a stain gives a rough idea to a researcher that the desired protein is present (based on size, mobility etc) and whether to go for immunodetection especially when expensive and limiting amounts of antibodies are available.
Topics: Western Blotting