Poor Quality Detergents Cause Artifactual Results

How Do Detergents Work?
The structure of detergents is key to its ability to function as a solubilization agent. Detergent molecules contain a polar head group from which extends a long hydrophobic carbon tail.
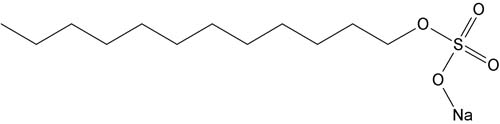
The amphipathic properties of the detergent molecules allows them to exhibit unique properties in aqueous solutions. The polar (hydrophilic) head groups interact with the hydrogen bonds of the water molecules and the hydrophobic tails aggregate resulting in highly organized spherical structures called micelles. At low concentrations, the detergents exist as single molecules or small aggregates and as the concentration increases micelles begin to form.
How Do Detergents Solubilize Proteins?
A wide range of detergents are routinely used to release, or solubilize, proteins from lipid membranes.
Biological membranes consist of phospholipids that are similar to detergents as they have the same amphipathic properties. The phospholipids have a charged polar head normally connected to two hydrophobic groups or tails. The phospholipids assemble as bilayers, with the hydrophobic tails between two faces of polar head groups.
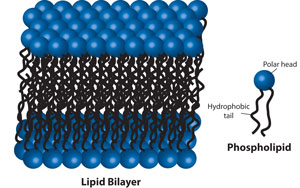
For biological membranes , proteins and lipids (i.e. cholesterol) are embedded in the bilayer forming the fluid mosaic model. The proteins are held in the lipid bilayer by hydrophobic interations between the lipid tails and hydrophobic protein domains. These integral membrane proteins are not soluble in aqueous solutions as they aggregate to protect their hydrophobic domains, but are soluble in detergent solutions.
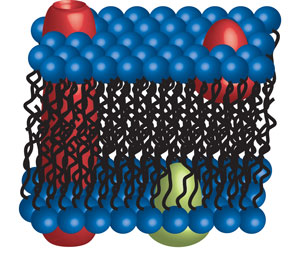
The proteins are released from lipid bilayers by detergents as the detergent micelles have similar properties as the lipid bilayer. The integral membrane proteins embed themselves in the detergent micelles protecting their hydrophobic domains from aggregation.
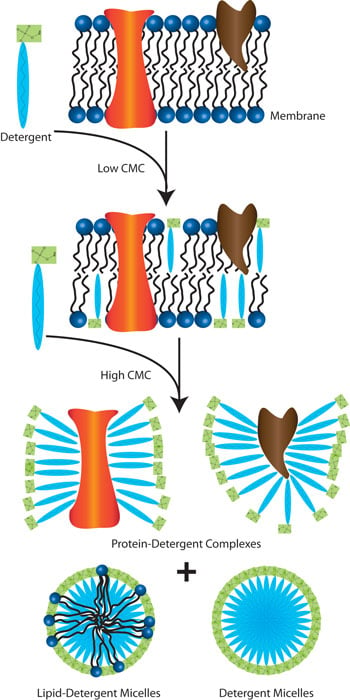
A schematic of how detergents solubilize membrane proteins is shown below. At low detergent concentrations, less than the detergent’s CMC, the detergent molecules insert themselves in the lipid membrane and begin partioning the lipid bilayer. At concentrations equal to, or higher than the detergent’s CMC, the lipid bilayer becomes saturated with detergent molecules and the lipid bilayer breaks apart. The resulting products are protein-detergent complexes, where the detergent hydrophobic regions bind to the protein hydrophobic domains protecting them from aggregations. In addition to these, detergent and detergent-lipid micelles are formed.
So Detergents Are Needed, How Can They Be Harmful?
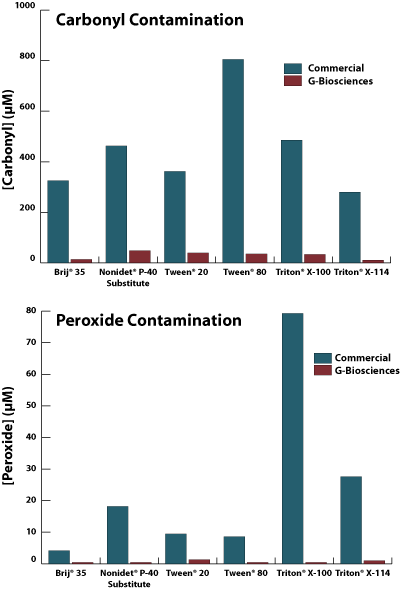
The manufacturing of commercial detergents, including non-ionic detergents, results in the presence of sulfhydryl oxidizing agents, peroxides, salts and carbonyl compounds. The level of these harmful reagents, particularily the peroxides and carbonyls (aldehydes) can increase over time as the product degrades.
The graph below shows a comparison between the aldehyde and peroxidelevels in G-Biosciences Proteomic Grade Detergents and commercial non proteomic grade detergents.
The oxidizing agents, peroxides and aldehydyes will react with protein amino acids causing changes to its primary structure, molecular mass and may also inhibit protein:protein interactions.
Proteomic Grade Detergents
G-Biosciences proteomic grade detergents are highly purified non-ionic detergents that contain low peroxide and aldehye contaminants. In addition they are supplied in small aliquots, sealed under inert gas, to prevent oxidization.
The Protein Man Review
Detergents are highly important for protein extraction, but the quality of the detergent is as important in the selction as is the type of detergent.
Proteomic grade detergents severly reduces the risk of artifactual modifications to the protein.
For more information on detergents, Download our Detergent Handbook
Do you have any tips for improved protein extraction?






