 Antibodies have become a great tool for identifying and characterization of different proteins in different model systems using the technique of immunohistochemistry and Western blotting. The precision and sensitivity of these molecules allow us to develop a clear-cut picture of molecular cascades and signalling pathways. The technique of characterization of proteins by immunohistochemistry is very old, however it requires elaborate standardization and rigorous scientific approach to select the right antibodies for the experimental need.
Antibodies have become a great tool for identifying and characterization of different proteins in different model systems using the technique of immunohistochemistry and Western blotting. The precision and sensitivity of these molecules allow us to develop a clear-cut picture of molecular cascades and signalling pathways. The technique of characterization of proteins by immunohistochemistry is very old, however it requires elaborate standardization and rigorous scientific approach to select the right antibodies for the experimental need.
Selection of an antibody for successful immunohistochemistry staining is a crucial step in getting the best result out of the experiment. To understand the function of proteins of interest and their spatio-temporal localisation, the epitope and the type of antibody should be considered carefully.
Primary Antibody Selection
Antibody specificity is one of the important aspects of the credibility of results obtained from immunohistochemistry. The clonality of the antibody is a deciding factor in antibody specificity. Polyclonal and monoclonal antibodies have their unique advantages and disadvantages in terms of immunohistochemistry and the choice of antibody.
|
|
Monoclonal Antibody |
Polyclonal Antibody |
|
Origin |
Multiple B-cell clones |
Single B-cell clones |
|
Specificity |
Multiple epitopes of the protein of interest |
Single epitope of the protein of interest |
|
Specificity rate |
lesser specificity |
Highly specific |
|
Advantages |
Resistant to antigen recognition after stringent processing methods Multiple epitope recognition allows signal amplification |
Lesser inter-lot variations Does not cross react with other proteins High signal to noise ratio |
|
Disadvantages |
Higher Inter-lot variations Low signal to noise ratio Less specificity |
Washing-off of the signal after stringent processing steps |
Table 1: Comparison of Polyclonal and monoclonal antibodies
Factors to be considered while choosing a primary antibody for an immunohistochemistry experiment:
- Species of the animal in which a primary antibody is raised: For multiplexing it is essential to check the species of animals so that the corresponding secondary antibodies do not cross react.
- The class (isotype) and/or subclass of the primary antibody should be known while performing the experiment. This is to make sure that the secondary antibody is raised against the same subtype or subclass.
- Labelling of the antibody: Certain primary antibodies that are developed to identify ubiquitously present or house-keeping proteins are directly labelled with the enzyme or fluorochrome. Therefore, it should be known before starting staining if your primary antibody is labelled or not.
Secondary Antibody Selection
Secondary antibodies are developed against the primary antibodies to allow their detection, signal amplification, sorting and purification of the primary antibody: antigen complexes.
Factors that determine the efficiency of a Secondary antibody:
- Specificity: The degree with which a secondary antibody binds with the primary antibody: antigen complexes determine its specificity. Non-specific binding with random antigens and tissue reduces the reproducibility of the results.
- Consistency: Reproducibility of the result is also another parameter of efficiency of the antibody. There should not be high variability across the lots of secondary antibodies providing a consistent result throughout the replicates.
- Sensitivity: The sensitivity of a secondary antibody is determined by its ability to bind with antigens which are present in the tissue in lesser quantity and require stronger binding and recognition of the epitopes easily.
Selection of secondary antibodies is as crucial as that of primary antibodies. It requires careful analysis of the primary antibodies and their development information. The points that are important to be considered for secondary antibody selection are:
- Host species: The host species in which the primary antibody is developed determines the host species of the secondary antibody. For example, if primary antibody is grown in mouse, the secondary antibody should be raised against mouse in other animals except mouse.
- Conjugates of secondary antibody: In order to visibly recognize the binding of antibody and antigen, secondary antibodies are conjugated with a reporter molecule which could be either an enzyme or a fluorochrome depending upon the application.
- Enzyme-linked secondary antibodies: Antibodies conjugated with enzymes such as alkaline phosphatase helps visualization of antigen through light microscope as it converts the substrate (for example DAB) into an insoluble-color product which can be imaged with conventional microscope easily.
- Fluorochrome-linked secondary antibodies: For fluorescent labeling, secondary antibodies are conjugated with fluorochrome dyes, such as AlexaFluor,which gets excited at a certain wavelength of light and results into emission of a visible spectrum captured through an epifluorescent or a confocal microscope.
- Selection of emission spectrum: While using secondary antibodies labelled with fluorochrome dyes, one should be careful about the excitation maxima and emission maxima of the dye. For multiplexed immunohistochemistry, this is the most crucial step for getting the trustworthy result. The emission spectrum of any two dyes should overlap or closely placed to avoid bleed through and false positive results.
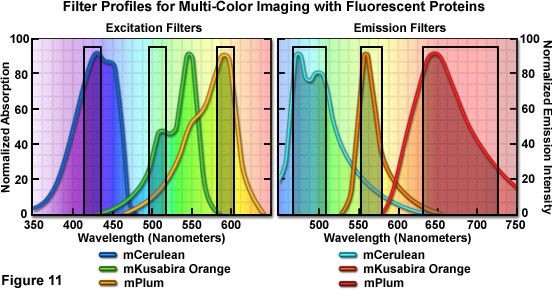
Figure: Diagrammatic representation of compatible spectrums of different dyes. Source: Olympus microscopy.
- Purity of the Secondary antibody: Lastly, it is suggested to use secondary antibodies purified by using affinity purification. This not only ensures the specificity of binding but also provide good results with lesser background and higher sensitivity. This is extremely important in case of identification of a low abundance or weakly detected proteins.






