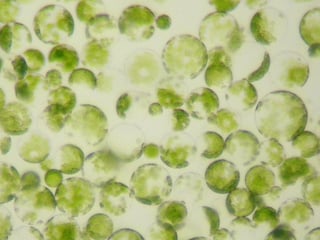
 Both protoplasts and spheroplasts refer to altered forms of plant, bacterial or fungal cells from which the cell wall has been partially or completely removed. These cells usually have all the other cellular components, except for the cell wall. When used in reference to bacterial cells, protoplasts may also refer to the spherical shape assumed by gram-positive bacteria while spheroplasts refer to the spherical shape assumed by gram-negative bacteria upon partial or complete removal of the cell wall. Cells with compromised cell walls assume a characteristic spherical shape to better withstand the rigors of its surrounding environment. They are also extremely sensitive to osmotic and mechanical shock.
Both protoplasts and spheroplasts refer to altered forms of plant, bacterial or fungal cells from which the cell wall has been partially or completely removed. These cells usually have all the other cellular components, except for the cell wall. When used in reference to bacterial cells, protoplasts may also refer to the spherical shape assumed by gram-positive bacteria while spheroplasts refer to the spherical shape assumed by gram-negative bacteria upon partial or complete removal of the cell wall. Cells with compromised cell walls assume a characteristic spherical shape to better withstand the rigors of its surrounding environment. They are also extremely sensitive to osmotic and mechanical shock.
Another main difference between protoplasts and spheroplasts is the number of membranes present. Protoplasts are bounded by a single membrane while spheroplasts have two - an inner membrane and an outer membrane.
Protoplasts and Spheroplasts: How are they formed?
The formation of bacterial protoplasts and spheroplasts can be induced in the laboratory. Since cell walls are composed of a variety of polysaccharides, viable protoplasts can be prepared by weakening the primary stress-bearing layer of the cell wall (peptidoglycan) using the appropriate enzymes. To accomplishing this purpose, researchers may either use mechanical or enzymatic methods.
Mechanical Method
In the past, protoplasts were isolated using mechanical methods which were labor-intensive, tedious and inefficient. Additionally, these methods were restricted to certain tissues with vacuolated cells and produced extremely low yields and barely viable protoplasts. For these reasons, most researchers now prefer the enzymatic method, except in cases where the enzymes can have a damaging effect on the resulting protoplasts.
Enzymatic Method
For plant cells, cellulase, pectinase, and xylanase can be used to break down the cell walls while lysozome (+EDTA) can be used to produce protoplasts from gram-positive bacteria. For fungal cells, using chitinase will do the trick. Similarly, spheroplasts can be prepared from gram-negative bacteria using procedures similar to those used in preparing protoplasts.
Upon the degradation of the peptidoglycan, the bacteria loses all its ability to control its response to the differences in the ionic concentration between its internal and external environment, so it becomes extremely sensitive to osmotic stress during and after the digestion of the cell wall.
For this reason, it should be stored in isotonic solution to prevent the plasma membrane from rupturing or shriveling. Maintaining the optimal ionic balance will also ensure the successful transformation of the bacteria into the desired form.
Protoplasts and spheroplasts can also occur naturally. In this case, they are referred to as L-forms (also L-phase bacteria, L-phase variants and cell wall-deficient (CWD) bacteria). Bacillus, Clostridium, Haemophilus, Pseudomonas, Staphylococcus and Vibrio are known to produce L-forms in nature.
Due to the absence of a defining cell wall, the morphology of L-form bacteria is very much different from the strain from which it was derived. Interestingly, while L-forms can be derived from both gram-positive and gram-negative bacteria, it is always detected as gram-negative when subjected to a gram stain test.
Some Useful Applications
Protoplasts and spheroplasts are valuable research tools and can be used in a wide range of applications.
Protoplasts
- Study of membrane biology, particularly in analyzing uptake and transport processes
- Somaclonal variation in plant tissues
- DNA transformation, especially in the creation of genetically modified organisms
- Plant breeding, specifically in the generation of somatic hybrids in tissue culture
- In Fluorescence Activated Cell Sorting (FACS), specifically in isolating certain cell types for further investigation
Spheroplasts
- Antibiotic recovery, particularly for antibiotics that inhibit cell wall biosynthesis
- For studying the function of bacterial ion channels using the “patch clamp” technique
- Transfection of animal cells
- Facilitation of cell lysis
Photo by Mnolf
Related Blog Posts






