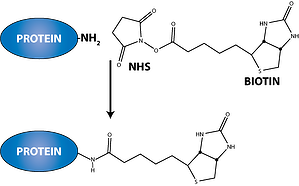
 Biotinylation, also known as biotin tagging/labeling, is the process of attaching biotin to a protein molecule and other macromolecules. Considering its high affinity to avidin and streptavidin (with a dissociation constant of around 10 -15 M, biotin-avidin/streptavidin interaction is one of the strongest non-covalent interactions existing in nature), its high specificity and fast on-rate, this technique is perfectly suited for isolating and purifying proteins and protein complexes, as well as identifying protein-protein interactions and post-translational events.
Biotinylation, also known as biotin tagging/labeling, is the process of attaching biotin to a protein molecule and other macromolecules. Considering its high affinity to avidin and streptavidin (with a dissociation constant of around 10 -15 M, biotin-avidin/streptavidin interaction is one of the strongest non-covalent interactions existing in nature), its high specificity and fast on-rate, this technique is perfectly suited for isolating and purifying proteins and protein complexes, as well as identifying protein-protein interactions and post-translational events.
Additionally, the biotin molecule’s relatively small size and the presence of a valeric side chain makes it ideal for tagging proteins and macromolecules. Since biotin is significantly smaller than globular proteins (molar mass = 244.31 g mol-1), they can be fused to a single protein molecule without interfering with its natural functions. Biotin also possesses an easily derivatized valeric side chain that can bind to reactive moieties without causing any adverse effects to its avidin-binding function.
Biotin tagging or labeling is extremely useful in protein research and can be used in a number of applications, including enzyme-linked immunosorbent assay (ELISA) and Western blot analysis. It can also be used for immunohistochemistry, immunoprecipitation, cell surface labeling, and flow cytometry. In addition, there is a very strong indication that biotinylation can also be used for examining protein-protein interactions and characterizations in living cells.
Biotin Labeling Methods
Biotinylation can be performed through chemical and/or enzymatic methods. While chemical methods are relatively more flexible and can be performed both in vivo and in vitro without affecting the protein’s structure, stability or function, the uniformity of the number of biotin molecules added cannot be guaranteed. There is also a risk that the binding sites will be inactivated due to the modification of certain lysine residues. On the other hand, enzymatic methods usually generate products with greater uniformity and can be cell-compartment specific.
There is a wide selection of biotinylation reagents available to suit your particular application. Most of the reagents provide functional group specificity (i.e., targets primary amines, sulfhydryls, carboxyls, carbohydrates). Photoreactivable reagents (reagents that react nonspecifically when exposed to ultraviolet light) are also available. These reagents are extremely useful when the functional groups (primary amines, sulfhydryls, carboxyls, carbohydrates) are not available for labeling and/or if the researcher wishes to activate biotinylation during an experiment by merely adjusting the existing conditions (e.g., exposing the reaction to ultraviolet light at specific times or conditions).
Why Consider Biotinylation?
There are a number of reasons why biotinylation is ideal for protein purification. Here are some of them.
- It allows purification of protein under extreme conditions. Biotin’s high affinity for avidin/streptavidin allows protein purification to proceed even under extreme environments (e.g., extreme temperature and pH conditions, the presence of organic solvents and other denaturing agents) without putting the tagged proteins at risk for early elution.
- It reduces background binding. The small number of naturally biotinylated proteins reduces nonspecific background binding which is commonly observed when using eluted affinity tags.
- There is a lower risk for cross-reaction. Unlike antibodies that can potentially cross-react with multiple species, there is a greatly reduced risk of cross-reaction when using biotinylated proteins.
- It increases the sensitivity of detection. Multiple biotin moleculescan be conjugated or fused to a protein of interest, thereby increasing the sensitivity of detection.
- You can use modified forms of avidin to suit your particular application. There are several forms of avidin that can be used for the elution of bound biotinylated proteins under native condition. These modified forms, which have lower binding affinities for biotin, can be used to purify tagged protein complexes and provide better resolution and identification of purified proteins.






