 Detergents play an extremely critical role in cell lysis and protein extraction. With their unique amphipathic nature (they possess a polar head group on one end that interacts with the hydrogen bonds of water molecules and a long hydrophobic carbon tail which aggregate to form micelles on the other end), this class of molecules can easily disrupt the hydrophobic-hydrophilic interactions between biological molecules in aqueous solutions.
Detergents play an extremely critical role in cell lysis and protein extraction. With their unique amphipathic nature (they possess a polar head group on one end that interacts with the hydrogen bonds of water molecules and a long hydrophobic carbon tail which aggregate to form micelles on the other end), this class of molecules can easily disrupt the hydrophobic-hydrophilic interactions between biological molecules in aqueous solutions.
This unique property makes detergents the most logical choice for lysing cells, solubilizing membrane-bound and/or recombinant proteins, and preventing non-specific binding in affinity purification and immunoassay procedures. Moreover, biological detergents can also be used for DNA and RNA extraction, liposome preparation, and the prevention of reagent and analyte precipitation.
How Do Detergents Work? Taking a Closer Look
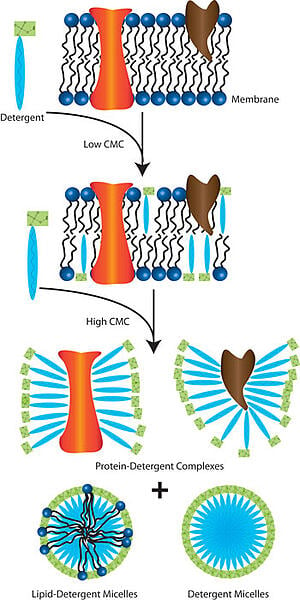
Basically, detergents and biological membranes are structurally alike since they are both made up of phospholipids with polar heads and hydrophobic tails. The phospholipids are usually arranged in bilayer sheets, with the polar head group exposed to the exterior and interior aqueous environments and the hydrophobic tails sandwiched between them. Embedded between the lipid bilayer are the proteins and lipids (e.g., cholesterol).
In aqueous solutions, integral membrane proteins aggregate to protect their hydrophobic domains, rendering them insoluble as a result. To release the proteins from the lipid bilayer, you need to use an appropriate detergent to solubilize your protein of interest and stabilize the folded protein to maintain functionality. It is also important that you choose one that does not interfere with your downstream applications.
Since detergent micelles exhibit similar properties as the lipid bilayer, detergent molecules can penetrate the lipid bilayer at concentrations less than the detergent’s critical micelle concentration (CMC), and compromise the integrity of cell membranes. As the concentration increases, more detergent molecules insert themselves in the lipid bilayer. The lipid bilayer finally breaks apart at concentrations equal to, or higher than the detergent’s CMC, resulting in cell lysis and the formation of protein-detergent and lipid-detergent complexes, and detergent micelles.
Detergent Selection: How to Select an Appropriate Detergent
When choosing which detergent to use for your application, the first thing you need to consider is the nature of the hydrophilic group. Using this as a basis, detergents can be classified as anionic, cationic, non-ionic and Zwitterionic or ampholytic.
Anionic and cationic detergents are known to modify the structure of a protein, are sensitive to pH and ionic strength, and can interfere with most downstream applications. Thus, they are generally considered as harsh biological detergents. On the other hand, non-ionic detergents are milder and allow proteins to retain their native structure. They are also less likely to cause protein denaturation. Most non-ionic detergents are classified as polyethers, bile salts, and glycosidic detergents.
Zwitterionic detergents are unique since they display several characteristics of both ionic and non-ionic detergents. They are less denaturing compared to ionic detergents but are more efficient in disrupting protein-protein bonds than non-ionic detergents.
Why Use Proteomic Grade Detergents?
While detergents can be used to facilitate cell lysis and membrane protein extraction effectively, not all detergents are created equal. Some commercial detergents may contain harmful reagents such as sulfhydryl oxidizing agents, salts, peroxides and carbonyl compounds (aldehydes) that may increase the risk of artifactual protein modifications. These compounds may react with protein amino acids and alter the primary structure and molecular mass of your protein of interest. Additionally, certain protein-protein interactions may also be inhibited.
Here are some tips to protect the integrity of your results when using these detergents:
- Always use proteomic grade detergents that contain the lowest amounts of contaminants (i.e., peroxide and aldehyde compounds, metal ions).
- Make sure your chosen detergents are supplied in small aliquots and sealed under inert gas to prevent oxidation.
- Choose detergents with low conductivity (ideally > 50μS conductivity).






