When it comes to solubilizing and denaturing proteins prior to isoelectric focusing and 2-D gel electrophoresis, most researchers choose urea. This is not surprising since this mild chaotropic agent can completely disrupt the interactions between protein molecules and increase the efficiency of protease activity in protein digestion while effectively preventing proteins from aggregating and/or precipitating. In addition, urea is also used for renaturing proteins from previously denatured samples.
 Despite its popularity as an effective protein denaturant, using urea solution in digesting proteins comes with a particular challenge – it increases the risk of carbamylation. Why should this concern you? Here are some reasons why carbamylation can be detrimental to your experiment and why it should be avoided at all cost.
Despite its popularity as an effective protein denaturant, using urea solution in digesting proteins comes with a particular challenge – it increases the risk of carbamylation. Why should this concern you? Here are some reasons why carbamylation can be detrimental to your experiment and why it should be avoided at all cost.
- Aside from blocking the N-terminal ends of protein molecules, carbamylation interferes with protein characterization and makes them unavailable for further use.
- It aggressively attacks the side chains of arginine and lysine residues and renders the protein unsuitable for enzymatic digests.
- It hinders the enzymatic digestion of proteins and adversely affects the identification and quantification of proteins in MS analysis.
- It confounds the results obtained from peptides with unexpected retention times and increases sample complexity.
- It reduces ionization efficiency and negatively affects the signal intensity of detected peaks.
- It induces a change in the isoelectric point of proteins and leads to artifactual results.
- It may affect the results of in vivo carbamylation studies.
What Causes Carbamylation?
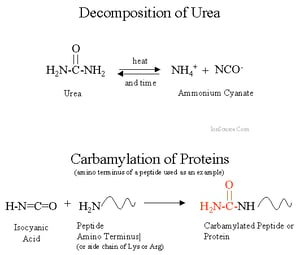
By definition, carbamylation refers to the post-translational modification which alters the structural and functional properties of proteins. This is brought about by the non-enzymatic reaction between isocyanic acid and the free amino groups.
In aqueous solutions, urea exists in equilibrium with ammonium cyanate. It is important to note that (1) urea in solution dissociates spontaneously producing cyanate and ammonium ions, (2) it degrades to isocyanic acid (CNOH), the form that actively reacts with protein amino groups and induces carbamylation, and (3) the rate at which urea dissociates and the extent of carbamylation depends on the temperature, pH, and incubation time.
Preventing Carbamylation: Some Useful Tips to Consider
Nothing can change the fact that urea in the presence of heat and proteins will always lead to carbamylation. You can use the highest grade urea available and still run the risk of carbamylation.
Thankfully, there are several ways to protect peptides from carbamylation. Here are some effective strategies that can be used to accomplish this goal.
- Always use freshly prepared urea-based reagents when conducting proteomic procedures. Use high-quality dry urea-based pre-mixed and ready-to-use buffers from credible sources and observe extreme caution in preparing your samples. Note: Store solid material at room temperature and keep handling to a minimum.
- Remove urea from the protein sample prior to digestion by employing dialysis and/or fast reversed phase chromatography or by using spin columns.
- Maintain the sample at a relatively low temperature to decrease the rate at which urea decomposes. Avoid heating urea-containing buffers above 37oC to reduce the risk of carbamylation.
- Deionize the urea solution using an ion exchange resin to reduce cyanate concentration.
- Acidify urea solution (100 mM HCl) to hinder the formation of isocyanic acid.
- Use reagents such as ethanolamine, ethylenediamine, methylamine, and Tris-HCl to minimize protein/peptide carbamylation. These reagents help by scavenging for cyanate molecules thereby making them unavailable for peptides.






