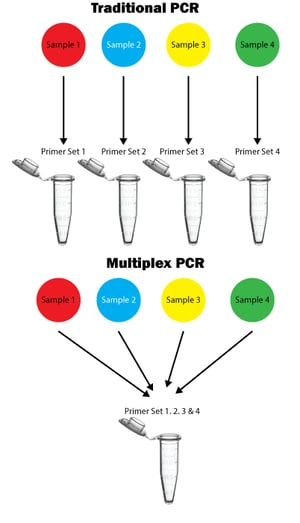
 Multiplex PCR is a technique used to amplify multiple target sequences in a single PCR reaction using multiple primer sets. Multiplex PCR was first reported by Chamberlain et al. in 1988. This method is used to detect deletions, polymorphisms, mutations, etc., This method is also used to detect different viral, bacterial and other pathogens in a single tube. This method consumes less time and effort in obtaining the results, but problems posed during multiplex PCR include poor sensitivity, specificity, and problems in amplification of some specific targets.
Multiplex PCR is a technique used to amplify multiple target sequences in a single PCR reaction using multiple primer sets. Multiplex PCR was first reported by Chamberlain et al. in 1988. This method is used to detect deletions, polymorphisms, mutations, etc., This method is also used to detect different viral, bacterial and other pathogens in a single tube. This method consumes less time and effort in obtaining the results, but problems posed during multiplex PCR include poor sensitivity, specificity, and problems in amplification of some specific targets.
BASIC PARAMETERS:
Primer design
Design of primers is one of the important parameters to be considered in multiplex PCR. The presence of more than one pair of primer results in either the formation of primer dimers or undesirable amplification products. Primer pairs used should have similar annealing temperatures and amplification efficiency. Primer pairs are tested by aligning in BLAST program and by trial and error method they are tested to avoid undesirable results.
Primer length should be 18-22 bases. Optimal melting temperature Tm is around 55-60°C. In the case of high GC content, Tm should be 75-80°C. Primers are designed in such a way that they have high specificity and sensitivity. Primer concentration should be optimized (0.04 µM-0.6 M) to avoid uneven amplification of products.
Optimization of reaction conditions
General optimal conditions are 0.2mM dNTP mix and 1.5 mM -2 mM MgCl2 concentration. DNA template concentration at 30-500 ng/25 µl reaction mix and Taq polymerase enzyme at 2U /25 µl reaction give better results. All these parameters can be adjusted accordingly.
PCR setup
Individual PCR reactions are optimized first by adjusting cycling conditions, PCR buffer and additives using a single primer set. Followed by setting multiplex PCR with all the chosen primer pairs and optimized PCR conditions in a single tube.
PCR gel analysis
Depending on the size of the PCR product, the sample is run on an agarose gel or polyacrylamide gel. Amplification products are analyzed on 6-10 % polyacrylamide gels if the distance between the products is less and amplification products above 400 bp are analysed on 3% agarose gels with a distance of 30-40 bp between each product. Running a gel overnight results in diffused bands and weak bands are hardly visible after long runs.
Troubleshooting
- All PCR products are weak: Use longer extension times. The decrease of extension temperature by 5°C. Decrease annealing temperature step-wise by 2°C. Optimize Taq polymerase concentration.
- Only short PCR products are weak: Increase Buffer concentration to 1.5x - 2x. Decrease both annealing and extension temperatures. Increase primer concentration by 0.05 µM increments gradually for weak loci.
- Only Long PCR products are weak: Decrease buffer concentration to 0.7x - 0.8x. Increase both annealing and extension temperatures. Increase primer concentration.
- Non-specific PCR products: If the PCR products are longer than expected then increase buffer concentration to 1.5x -2x. If the PCR products are shorter than expected then decrease the buffer concentration to 0.7x -0.8x. Increase annealing temperature gradually. Decrease template concentration and Taq polymerase concentration. Increase MgCl2 concentration to 3-12 mM gradually.
Advantages of Multiplex PCR:
- The presence of internal controls in a multiplex PCR helps in the detection of false negatives.
- Multiplex PCR is a cost-effective method and less time-consuming. Low quantities of template and expensive polymerase enzyme and reagents can be conserved by this method.
- Quantitation of template is possible by comparing with a standard template by comparing the number of cycles and minimum inhibition of product doubling.
References
- Chamberlain, J. S., Gibbs, R. A., Ranier, J. E., Nguyen, P. N., & Caskey, C. T. (1988). Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic acids research, 16(23), 11141-56.
- Elnifro, E. M., Ashshi, A. M., Cooper, R. J., & Klapper, P. E. (2000). Multiplex PCR: optimization and application in diagnostic virology. Clinical microbiology reviews, 13(4), 559–570.






