Diagnostic field has been a progressing area with multiple tools identified for specific proteins detection and their quantification. Diagnosis in pathology or clinical set up has multiple numbers of challenges owing to time constraint, increasing numerosity and complexity of the detection methods, less amount of sample and so on, which is essentially important for disease identification, refining of pathological interventions and development of an appropriate prognosis. Pathological detection is most commonly performed by utilizing antibody-antigen interactions (by deposition of a visible product or fluorochrome based detection). The standard approach is to perform one or two protein detection at a time on the serial sections, which is cumbersome if the sample availability is low and the proteins to be detected are high in number. More often fluorescent reporters are used for both detection and quantitation methods such as image cytometry, flow cytometry, confocal microscopy, etc. using live tissue or slightly fixed tissue, while the conventional immunohistochemistry involves heavily fixed tissue sections. Recently, in the wake of developing user-friendly and multiple assays on formalin fixed, paraffin embedded (FFPE) specimens are being developed and gaining ground, known as Multiplexing or multiplex immunohistochemistry (mIHC), which allows use of more than three different stains performed on one sample/ slide.
The cardinal strategy to carry out multiplexing IHC on the same section at one point of time requires the use of multiple animal specific secondary antibodies linked with various reporters against antibodies raised in different animals (rabbit, goat/sheep, rat, and three to four mouse isotypes). Although, there are a finite number of antibody sources, hence it is certain that developing a common multiplexing strategy to stain antibodies developed in the same species with different colors is difficult. However, there could be three possible approaches:
- First bleach the directly conjugated primary antibodies and then add another set of antibodies
- Block the epitopes of already labelled antibodies followed by a second round of staining
- Remove previously labelled antibodies from the sections after staining and imaging, followed by another set of labelling.
These methods for multiplexing has few disadvantages including limited diffusion, steric hindrance, loss of antigens etc despite of being time saving and sample saving. The topography of multiplexing may take a new shape with the introduction of "next generation IHC” coupling isotope-tagging and in situ mass-spectrometry detection. Along with that, the availability of specialised barcode labelled probes/reporters, including antibodies, opens up a vast and comprehensive domain of multiplexing with the NanoString technology.
There are two types of multiplexed staining methods:
Brightfield multiplexing:
For brightfield multiplexing, FFPE (formalin fixed, paraffin embedded) tissues are probed with various enzyme/chromogen pairs resulting into a bright light visible chromogenic deposition. There are various chromogenic substrates are available which produce a solid color precipitate when acted upon by the attached enzyme. These precipitates can be visualized under a bright field microscope. The chromogens are generally tagged with horseradish peroxidase (HRP; DAB, DAB + Ni, AEC and VIP) or alkaline phosphatase (AP; NBT/BCIP). These multiple staining can be counterstained with dyes such as methyl green or haematoxylin to get an enhanced background.
Though this method is useful to differentiate between cell types, but it is exigent when the targeted proteins are co-localized within the cells. Hence before going ahead with brightfield multiplexing, several other factors are needs to be considered for Brightfield multiplex IHC. Essentially, the primary antibodies used for multiplexing has to be chosen carefully to avoid cross reactivity (conjugated with HRP or AP). This problem can be partially avoided by following sequential staining methods. However, the procedure is laborious and leads to possible tissue degradation with successive assays. Also, a possibility of chromogenic overlap is undeniable. Considering all these, multiplexed chromogenic IHC is a worthy tool utilised in pathological advancements, but the multiplexing beyond 3 targets is practically difficult.
|
Substrate (Chromogen) |
Enzyme |
Product colour |
|
DAB |
HRP |
Brown |
|
DAB+ Nickel |
HRP |
Black |
|
AEC |
HRP |
Red |
|
VIP |
HRP |
Purple |
|
Nova Red |
HRP |
Deep red |
|
TMB |
HRP |
Blue |
|
NBT/BCIP |
AP |
Deep blue |
|
Vulcan Fast Red |
AP |
Red |
|
Vector Black |
AP |
Black |
Table1: Chromogens used in multiplex IHC (brightfield)
Fluorescent multiplexing:
Fluorescence mIHC has an upper hand over brightfield mIHC as it has light emission with multiple spectral peaks against a dark background. Fluorophores based mIHC can be achieved by either direct or indirect labeling. In case of direct labeling, the fluorophores is conjugated with the secondary antibody that specifically binds with the primary antibody. Indirect labeling has additional amplification steps which can be achieved by either binding of multiple secondary antibodies to a single primary antibody or by using amplification complexes such as avidin–biotin complex (ABC). The important factor to be considered in fluorescence multiplexing is the choice of filter cubes. The microscopic source must have a bright light source as well as paired excitation/ emission filters corresponding with the fluorophores used.
Also, it is crucial to note the potential likelihood of co-localization. The analysis might give false positive results if the colours are not chosen carefully with respect to colocalized proteins, such as if the colocalization of red and green provides a degree of yellow then the non-colocalized protein should not have yellow fluorophore. The most successful multiplexing is obtained by indirect labeling with primary-specific conjugated secondary antibodies, which sometimes provide a constraint in terms of accommodating one species of primary (e.g. rabbit, mouse, or goat) for each target of interest tagged with a specific secondary antibody coupled with a specific fluorophore. Apart from this, primary antibodies directly conjugated with fluorophores can be utilized as a great tool for multiplexing. However these methods are labor intensive and difficult to perform in a normal lab set up. An alternative approach has been developed in order to nullify this disadvantage known as multi-epitope-ligand-cartography (MELC), a fluorescent multiplex in situ strategy which aids in providing simultaneous protein expression visualization.
Another interesting tool to circumvent the complications of low signal in multiplexing is by amplifying it using Tyramide signal amplification (TSA) method which allows signal amplification by binding with the epitope of the antibody in a very specific manner. This step overcomes the restriction of use of similar species antibodies as individual primary/secondary antibody complexes can be removed after serial IHC, although the covalent signal stays intact for imaging.
TSA method can be employed quantitatively and is driven by enzymatic reaction, closely similar to that of chromogenic staining. But unlike chromogenic detection, TSA supports serial staining and fluorescent multiplexing.
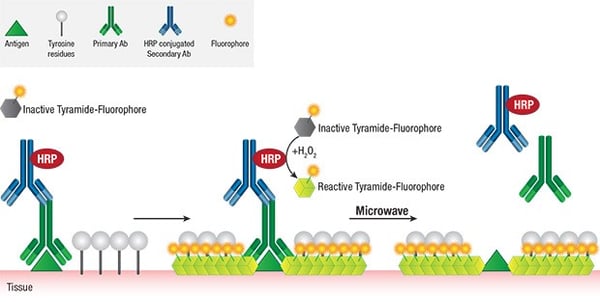
Figure 1: Overview of Tyramide signal amplification (TSA). Source: Cell signalling technologies
|
Fluorophore |
Excitation wavelength (λ) nm |
Product colour (λ) nm |
|
Cyanine 680 |
669 |
688 |
|
Cynanine 5 |
648 |
667 |
|
Cynanine 3.5 |
581 |
596 |
|
Cynanine 3/ TMR |
550 |
570 |
|
Fluorescein |
494 |
517 |
|
Coumarin |
402 |
443 |
Table 2: List of fluorophores used in multiplexed fluorescent IHC.
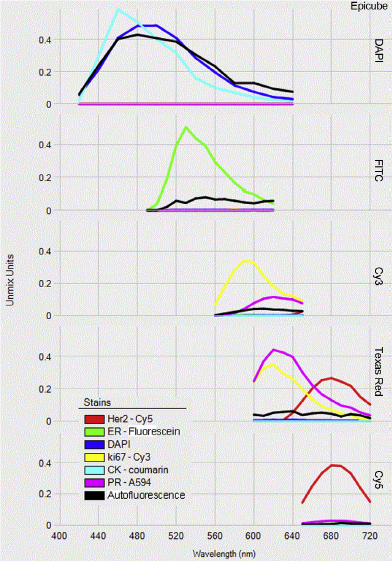
Figure 2: Spectral library from a multiplexing IHC assay. Source: E.C. Stack et al. / Methods 70 (2014)
Advantages of multiplexed immunostaining:
- It allows maximal data harvesting per sample (tissue section), which is crucial when the availability of sample is low.
- Unlike other multiplex approaches such as next generation sequencing, PCR, mass spectrometry, etc, multiplex immunostaining gives an edge to understand co-expression and spatial distribution of many targets without disturbing the tissue integrity.






