 In the radioimmunoassay, high specific radioactive antigens are used as tracers. Iodinated proteins are used to study hormonal interaction with target tissue and immunoglobulin metabolism. The chloramine-t procedure is the oldest method used to iodinate proteins and peptides directly with 125 I, but it is observed that there is a loss of activity of proteins iodinated by this method. The disadvantage of this method is difficulty in iodination of proteins lacking tyrosine residues.
In the radioimmunoassay, high specific radioactive antigens are used as tracers. Iodinated proteins are used to study hormonal interaction with target tissue and immunoglobulin metabolism. The chloramine-t procedure is the oldest method used to iodinate proteins and peptides directly with 125 I, but it is observed that there is a loss of activity of proteins iodinated by this method. The disadvantage of this method is difficulty in iodination of proteins lacking tyrosine residues.
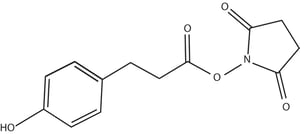
Iodinated N-hydroxysuccinimide ester of 3-(4-hydroxyphenyl) propionic acid, also known as Bolton-Hunter reagent or SHPP, is successfully used to iodinize proteins that lack tyrosine residues. In this method, the protein is covalently coupled with the acylating agent, where the acylating agent reacts with protein side chains at lysine residues. This method is more suitable for proteins with few or no tyrosine residues. Also suitable for proteins susceptible to oxidation. This method has many applications including target labeling of cytosolic proteins, membrane proteins, viruses, cell lysates and compounds like Gentamicin. Most importantly labeling of target antigens and hapten derivatives of high specificity for use in radioimmune assay.
Reagents required
- Bolton Hunter Reagent
- Buffer 1- 0.1 M Borate buffer (pH 8.5)
- Buffer 2- 0.2 M Glycine+ 0.1M Borate buffer (pH 8.5)
- Buffer 3- 0.05M Phosphate buffer (pH 7.5) with 0.25% gelatin
- Buffer 4- EBSS- Earle’s Balanced salt solution
Procedure
Radiolabeling the protein by conjugation with Bolton-Hunter reagent
- 5 µg of protein in 10 µl of 0.1 M Borate buffer pH 8.5 was added to 0.2 µg of Bolton-Hunter reagent (Bolton-Hunter-Reagent-SHPP) and the reaction mixture is kept on stirring for 15 min at 0°C.
- Generally, 3-4 mol of labeled ester was reacted per mol of protein.
- Unconjugated ester is removed by adding 0.5 ml of 0.2 M Glycine in 0.1 M Borate buffer pH 8.5 for 5 min at 0°C.
- Excess amino groups from Glycine buffer conjugates with the unreacted Bolton Hunter reagent making it unavailable to iodine carrier protein of the next buffer (gelatin).
- Radiolabelled protein is separated from the other labeled products, glycine conjugate, etc., by using a gel filtration column. SpinOUT™ GT-600 spin column is pre-equilibrated with 0.05M Phosphate buffer pH 7.5 with 0.25% gelatin and then eluted with the same buffer.
Radiolabeling the cell lysates by conjugation with Bolton-Hunter reagent
- 200 µl of cell lysate in EBSS was adjusted to pH 8.5 with the addition of Sodium carbonate.
- Bolton-Hunter reagent was added to the cell lysate to iodinize free amino groups in the cell lysate.
- Removal of unconjugated ester is done by adding 0.5ml of Buffer 2 followed by filtration column or dialysis.
Radiolabeling the membrane proteins by conjugation with water-soluble Bolton-Hunter reagent
Radiolabelling of Membrane proteins is challenging as most of the reagents used to iodinate cytosolic proteins can’t penetrate membrane proteins. When iodinizing agents like radioactive Iodide with lactose peroxidase and glucose oxidase, Chloroglucouril are used, either only 0.1% tyrosine are labeled or instead of membrane proteins only cytosolic proteins are labeled.
Water-soluble Bolton Hunter reagent (Sulfo-SHPP-Water-Soluble) can be used to iodinate membrane proteins to higher specific activity avoiding labeling of cytoplasmic proteins.
Reagents:
Buffer 5- Phosphate Buffered Saline (PBS, 7.4 pH)
Buffer 6-10 mM HEPES, 0.15 M NaCl, 1 mM EDTA (7.4 pH)
Buffer 7- 10 mM HEPES, 2% Triton X-114 (7.4 pH)
- Radio iodination of cells is carried out by adding 100-150 µl of 125 I-sulfo SHPP Bolton Hunter reagent in 5 ml DPBS to pre- washed confluent HF Cells (PBS) from 100 mm dish and incubated for 30 minutes on ice.
- The reaction was terminated by adding 1ml of 1mg/ml Lysine in DPBS. Cells were then washed thrice with DPBS (Buffer 5).
- Membrane proteins are extracted in four different fractions. 2ml of Buffer 6 is added to intact cells and incubated at 22°C for 10 min.
- Cells were scrapped and centrifuged. The first fraction of membrane proteins were collected in the supernatant.
- The remaining cell pellet was incubated with 100 µl of Buffer 7 for 10 min and centrifuged to remove insoluble material.
- To the soluble fraction, 30 µl of 4M NaCl fraction is added and incubated for 15 minutes at Room temperature followed by centrifugation for 1 minute.
- The soluble fraction separated into two phases. The second and third fractions of membrane proteins were collected from the detergent-free phase.
- An insoluble fraction from step 4 is dissolved in 100 µl of SDS sample buffer (Fraction 4).
- The first three fractions were pooled and Acetone is added to 90% to precipitate proteins.
- Precipitated proteins are suspended in 100 µl of SDS sample buffer and all fractions proceeded for further analysis by electrophoresis. After electrophoresis, gels were stained, dried and autoradiographed.
- Quantification of I125 is done by precipitating 10 µl of membrane protein fraction with 1ml of cold 10% Trichloroacetic acid and incubated on ice for 10 minutes.
- Precipitated proteins were collected on Whatman GF/C filters and washed with 2 ml of cold 10 % Trichloroacetic acid and analyzed by using Beckman gamma counter.
References:
- Bolton, A E, and W M Hunter. “The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent.” The Biochemical journal 133,3 (1973): 529-39.
- John J. Langone, Radioiodination by use of the Bolton-Hunter and related reagents, Methods in Enzymology, Academic Press, Volume 70, 1980, Pages 221-247.






