Routine H&E (Haemotoxylin and Eosin stains) and special staining comes especially handy when examining tissue structure and cell types and/or when looking for the presence of certain microorganisms in a sample. While both stains are used in histopathology laboratories, H&E stain is more commonly used by pathologists and researchers for investigating underlying cellular and tissue structures.
H&E stain’s simplicity, and ability to clearly elucidate the tissue’s basic morphology by staining nuclei and cytoplasm in different colors makes it ideal as a control for any type of histological work. Thus, it is used as a control for all immunohistochemical staining to show the tissue was correctly processed and contains no artifacts. It is virtually unheard of a pathologist making a diagnosis without looking at an H&E control slide in addition to whatever specific stains or IHC detection may have been used for that particular case.
H&E also serves as what is arguably the most popular background stain in immunohistochemistry (IHC). When using an antibody to detect a specific protein through IHC, a background stain such as H&E is used to simultaneously visualize the cells where the protein is being detected.
On the other hand, special stains are used when the pathologist/researcher wants to take a deeper look at the morphological profile of a particular tissue. In most cases, special stains are used when the pathologist/researcher wants to differentiate and/or identify components observed in tissue sections previously stained with H&E.
H&E Stain: How Does It Work?
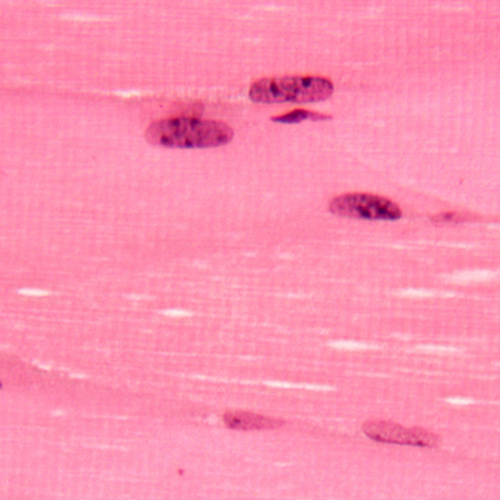
Figure 1. Skeletal muscle (MM) H&E. Striations clearly visible in cytosol
and heterochromatin clearly visible in nuclei.
H&E staining makes use of two dyes – hematoxylin (a natural basic dye extracted from the logwood tree) and eosin (an acidic dye that gives a bright pink color). This combination works perfectly together since the dyes stain different tissue elements – hematoxylin stains the acidic (basophilic) tissue components (e.g., nucleus, ribosome, rough endoplasmic reticulum) with a purplish blue color while eosin gives nearly all other (eosinophilic) components (e.g., cytoplasm, cell walls, extracellular fibers) a reddish or bright pink color.
While hematoxylin itself is not a good dye, when oxidized, it turns into hematein; which has the ability to form strong, colorful complexes with certain metals. The metallic salt (aluminum and/or iron) or “mordant” helps it bind to the nuclear chromatin. The metal salt used to form the complex will determine the color it will have; Alum-hematein complexes are red in acidic solutions at first, but turn blue/purple in slightly basic solutions during a process called blueing. Iron-hematein complexes tend to look bluer or even black. Other, less frequently used complexes exist (lead, tin, tungsten, copper) for different applications. However, alum formulations are by far the most popular, followed by iron ones.
Hundreds of different alum formulations have been created since its original creation in 1865. However, they can all be classified as either progressive, or regressive stains. Progressive stains are those where the intensity progressively increases with staining time. The process has to be interrupted at the right time in order to achieve the desired intensity. Regressive stains are designed to initially overstain the tissue, while allowing for a subsequent destaining process that gradually leads to the desired stain intensity. Both of these staining techniques allow for a customized, controlled staining intensity but require different procedures sometimes different formulations. What process or formulation is used tends to be a matter of personal preference and application.
In regressive staining, the slides containing the samples to be stained are submerged in the solution for about 5 minutes to saturate all the available binding sites. Excess stain is removed by a “differentiation” step. Here the slides are washed in an acidic solution composed of either dilute HCL or acetic acid in water or alcohol. How fast this acid wash works will vary greatly depending on whether HCL or acetic acid was used, its concentration, and alcohol content (more alcohol slower destaining). The pathologist will then destain until the intensity is adequate, which can sometimes be a subjective matter where personal preference comes into play. The slides are then “blued” by rinsing in tap water until the sections turn blue. While tap water usually provides the alkalinity needed for the bluing process, this varies by location and other alternatives such as “Scott’s water substitute” or ammonia solution can be used. To differentiate the nucleus from the non-nuclear components, the slides are counterstained with 1% eosin for 10 minutes. Finally, the slides are dehydrated in increasing concentrations of alcohol, cleared in xylene and mounted in mounting media for observation.
Staining in a progressive manner requires the same steps except that the acid differentiation is not needed. The process relies on stopping the staining process at just the right time for the intensity to be adequate. This will depend on the formulation of the stain as well as the type of sample (i.e. Frozen sections will stain much faster than paraffin embedded tissue).
Special Stains: What’s so Special About Them?
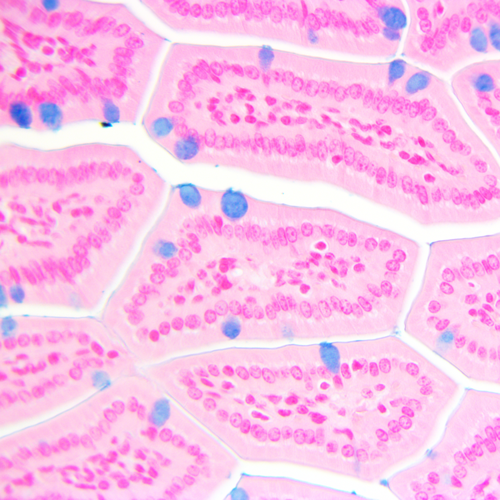
Figure 2. Alcian Blue PLUS with Nuclear fast red counterstain in small intestine section (MM)
showing blue mucopolysacharides and glycoproteins in goblet cells
By definition, special stains refer to a wide variety of alternate staining techniques and procedures used to provide more in-depth information on a particular sample. Rather than specific antibody binding, special staining techniques are based on simple chemical reactions such as acid-base chemistry and oxidation-reduction reactions.
By using special stains, you can get more detailed information on the presence and localization of certain substances (normal or abnormal) in cells or tissues, as well as the number of molecules present in the sample. Thus, these stains come especially handy when you need to detect the presence and identify microorganisms and/or specific tissue molecules (e.g. glycoproteins and/or glycosaminoglycans) in cancers and other pathological conditions.
There are a lot of special stains available. There are those that can be used for the detection of microorganisms (gram staining, giemsa stain, acid fast blue, acid fast green, etc.), connective tissues and lipids (toluidine blue, elastic stain, gomoris trichrome blue, gomoris trichrome green, Massons trichrome stain, etc.) and carbohydrates (mucicarmine stain, alcian blue, acid-schiff, alcian blue PAS). There are also special stains that can be used to detect minerals (iron stain, von Kossa stain, colloidal iron) and pigments in specimens.
Here are some things you may want to consider when working with special stains:
- Always use positive controls. Aside from helping determine if the stain is working, using positive controls also serves as a standard and will give you an idea of how the substance should look like.
- Beware of impurities. Special stains may contain colorless impurities that may interfere with the results.
- Use the right amount of stain. Stained specimen should be mounted in a medium that does not promote leaching or bleaching.
Related Blogs






