Monitor oxidative stress to determine the extent of cell damage using a colorimetric gluthathione assay.
What is oxidative stress?
Oxidative stress is caused due to an imbalance between production of reactive oxygen species (free radicals) and effectiveness of antioxidant defense. Reactive oxygen species (ROS) play a crucial role in cell signaling, however when the balance between ROS production and consumption is disrupted, it can lead to cell damage. Oxidative stress can cause damage to DNA, proteins and lipids. Reactive oxygen species are produced by electron leak from aerobic respiration by mitochondria. Enzymes like NADPH oxidases, xanthine oxidases, cytochrome P450 and other oxidases also produce ROS. There are enzymes and molecules in the body that serve as antioxidants such as superoxide dismutase (SOD), catalase, glutathione peroxidase and glutathione which removes ROS molecules from the living system.
How do levels of glutathione change during oxidative stress?
Reduced glutathione (L-g-glutamyl-L-cysteinylglycine), a key antioxidant present in animals, plants, fungi and bacteria provides reducing equivalents in form of free thiol groups. Glutathione exist in reduced (GSH) and oxidized (GSSG; glutathione disulphide) forms in cells and tissues, and the concentration of glutathione range from 0.5 to 10mM in animal cells. The majority (90-95 %) of glutathione exist in reduced form (GSH) in healthy cells. GSH provides reducing equivalents to antioxidant enzymes, hydroxyl radicals, ROS and is itself oxidized to GSSG; therefore GSH/GSSG ratio is critical indicator of the health of cell. During oxidative stress there is decrease in levels of GSH and increase in levels of GSSG and thus GSH/GSSG ratio decreases.
How do glutathione assays work?
Glutathione assays involve the measurement of reduced glutathione, oxidized glutathione and total glutathione. Thus with the help of a glutathione assay, GSH/GSSG ratio is determined and the level of oxidative stress can be measured. The most widely used assay for estimation of glutathione is the colorimetric assay. The assay involves carefully optimized enzymatic recycling method using glutathione reductase and Ellman’s reagent (DTNB). Glutathione reductase reduces GSSG to GSH at the expense of oxidation of NADPH. DTNB (5-5’-dithiobis [2-nitrobenzoic acid) reacts with GSH to form yellow color chromphore, 5-thionitrobenzoic acid (TNB) with absorbance maxima at 415 nm and GS-TNB. GS-TNB is further reduced to GSH and TNB by glutathione reductase and NADPH is oxidized in the process. This enzymatic recycling of GSH enhances the sensitivity of the assay. The glutathione concentration of unknown sample is measured by measuring the absorbance at 415 nm and comparing it with standard curve for GSSG, which is plotted new every time when glutathione quantification is done.
Glutathione colorimetric assay is rapid and can be used to measure concentrations of reduced glutathione (GSH), oxidized glutathione (GSSG) and total glutathione (GSH+GSSG) in wide range of samples such as, blood, plasma, serum, cultured cells and tissue.
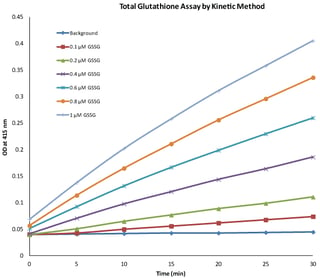
Adapted from G-Biosciences' Glutathione Assay
Important Research areas for Glutathione Assays
Oxidative stress has been implicated to play a role in ageing and many pathological diseases, including cancer, diabetes, cardiovascular disorders, artherosclerosis, Parkinson’s and Alzheimer’s . One of the ways in which oxidative stress can be monitored or measured is by checking the ratio of GSH to GSSG.
Glutathione assay is an indirect assay to determine redox state of cells.
Reduced Glutathione serve as potential therapeutic agent for several diseases. When GSH is administered as therapeutic agent, its metabolism or effect can also measured by glutathione assay.







