 What is a Protease Inhibitor?
What is a Protease Inhibitor?
If you want to protect your target protein from potential harm, there is one thing you should do – use a protease inhibitor.
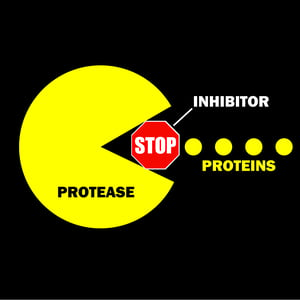
Protease inhibitors are biological and chemical compounds that deactivate protease activity. They also bind reversibly or irreversibly to active proteases in a solution.
A complete protease inhibitor protects protein samples from the digestive function of proteases, which begins in the isolation procedure.
As proteases are released naturally following cell or tissue lysis, these compounds preserve cell lysates and protein samples from imminent natural degradation. Also, they maintain the nature of the samples for subsequent experimentations.
Protease inhibitors can either be classified according to the type of protease they act upon, including serine proteases, cysteine proteases, aspartic proteases, and metalloproteases. They can also be classified according to their mechanisms of action, such as reversible inhibitors or irreversible inhibitors.
Reversible inhibitors bind to the protease through multiple non-covalent interactions and can be removed by dilution or dialysis. Irreversible inhibitors, on the other hand, alter the active site of its target through the covalent bond formation. Upon binding, the active site of the protease is changed so it can no longer perform peptide bond hydrolysis.
There are several common types of protease inhibitors, which include:
- AEBSF (4-3-(Aminoethyl)-benzenesulfonyfluoride hydrochloride)
- Aprotinin
- Bestatin
- EDTA/EGTA
- Leupeptin
- Pepstatin A
- PMSF (Phenylmethanesulfonyl fluoride)
Among these inhibitors, scientists typically rely upon AEBSF and PMSF to protect their protein samples.
Protease inhibitors are suitable for extracting and safeguarding various types of protein, including bacterial cells, mammalian cells, plant cells, and tissue extracts.
Due to the variety of protease inhibitors available, it can be difficult and time-consuming to choose the correct protease inhibitor for your application, and how to use it properly.
Nonetheless, it is still essential to understand how a protease inhibitor works, so you can protect the health and integrity of your protein samples and use them for future applications.
How does a Complete Protease Inhibitor Cocktail Work?
Before use, it is crucial to learn how a protease inhibitor operates.
A protease inhibitor works by reversibly or irreversibly deactivating the protease in the cell lysate and binds to the active site or modifies the structure. These actions prevent the hydrolysis of the protein sample and allow you to store your lysate for a more extended period, without it naturally degrading over time.
However, protease inhibitors are not the same. All inhibitors operate differently, so there may be times where you must determine the proteases to block before choosing the correct one.
Furthermore, since it is impossible to deactivate every known protease effectively, some scientists prefer to utilize ready-to-use complete protease inhibitor cocktails. They are ideal for lysing cells and homogenizing tissues without compromising the health and integrity of the samples.
Rely on G-Biosciences for Your Protease Inhibitor Needs
At G-Biosciences, we offer an https://www.gbiosciences.com/Protease-Protease-Inhibitor-Systems/Protease-Inhibitors. For more information about our complete protease inhibitor products, visit our website or contact us today.






