EDC is the most popular zero-length crosslinker for biochemical conjugations because it can efficiently form conjugates between two protein molecules, between a protein and a peptide, and between proteins and oligonucleotides, and with small molecules.
Direct EDC mediated crosslinking can be done without EDC becoming part of the final amide bond between the target molecules. Another advantageous quality of EDC is that it is water soluble and dissolves in aqueous buffer solutions, like most biological macromolecules. Therefore, EDC and its by-product, isourea, are dissolved into the reaction medium allowing easy purification of the crosslinked product with precipitation, chromatography, dialysis, or ultrafiltration.
For higher coupling efficiency and more stable amine-reactive intermediates, EDC crosslinking protocols often include N-hydroxysuccinimide (NHS) or its water-soluble analog (Sulfo-NHS). EDC, in conjunction with NHS allows, for 2-step coupling of two proteins without affecting the carboxyls of the second protein.
First, EDC activates carboxyl groups and forms an amine reactive O-acylisourea intermediate that spontaneously reacts with primary amines to form an amide bond and an isourea by-product. The O-acylisourea intermediate is unstable in aqueous solutions and failure to react with an amine will cause hydrolysis of the intermediate, regeneration of the carboxyls, and the release of an N-substituted urea. Therefore, it is necessary to quench the EDC activation reaction with a thiol-containing compound like 2-mercaptoethanol. EDC couples NHS to carboxyls, which forms an NHS ester that is considerably more stable than the O-acylisourea intermediate and allows for efficient conjugation to primary amines at physiologic pH.
2-Step Coupling with EDC & NHS
Materials Required
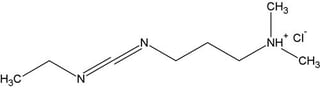
- EDC (1-ethyl-3-(3-dimethylamino) propyl carbodiimide, hydrochloride
- Conjugation Buffer 1
- 0.1M MES (4-morpholinoethanesulfonic acid), pH4.5-5 or G-Biosciences Optimizer Buffer-IV (Cat. # BKC-07)
- Conjugation Buffer 2: 1X PBS
- Protein #1 (1mg/ml), prepared in Conjugation Buffer 1
- Protein #2 (1mg/ml), prepared in Conjugation Buffer 2
- NHS of Sulfo-NHS
- Desalting column
We recommend SpinOUT™ GT-600, 3ml (Cat. # 786-171) - 2-Mercaptoethanol
- Hydroxylamine.HCl
Protocol
- Equilibrate the EDC and NHS to room temperature before opening.
NOTE: These are highly hygroscopic; failure to equilibrate may lead to poor cross linking. - Prepare 1ml of a 1mg/ml solution of Protein #1 in Conjugation Buffer 1.
- Add 0.4mg EDC and 0.6mg NHS or 1.1.mg sulfo-NHS and react for 15 minutes at room temperature.
- Add 1.2µl 2-mercaptoethanol to quench the EDC.
NOTE: At this stage the protein can be separated from excess 2-mercaptoethanol with a desalting column - Add an equimolar amount of Protein #2 compared to Protein#1 and allow to react at room temperature for 2 hours.
- Quench the reaction with the addition of hydroxylamine to a 10mM final concentration.
- Purify the coupled proteins using a desalting column.
We recommend our SpinOUT™ desalting columns.
Other related blog titles:
- Modifying Oligonucleotide 5'-Phosphates By EDC for Improved Coupling
- The 3 Types of Crosslinking Reagents and When to Use Them
- How To Determine Degree of Protein Labeling
- Biotin Labeling: Key Points to Selecting Your Biotin Agent






