Since accurate protein quantitation is essential to all experiments related to protein studies, different methods have been developed to measure the concentration of proteins in a given assay. Some of the more traditional methods of total protein quantitation include the measurement of UV absorbance at 280 nm (A280), Bicinchoninic acid (BCA) and Bradford assays, and other alternative methods such as Lowry and other novel assays.
Since proteins absorb light at a specific wavelength, measurement can be obtained using a spectrophotometer. Specifically, the amino acids tyrosine and tryptophan have a very specific absorption at 280 nm, allowing direct A280 measurement of protein concentration.
UV absorbance at 280 nm is routinely used to estimate protein concentration in laboratories due to its simplicity, ease of use and affordability. Measurements are quick and highly reproducible since there is no need for incubation. In addition, this method also requires an extremely small sample volume since modern spectrophotometers use a sample retention system during the measurement.
However, care should be taken to ensure that the protein sample does not contain any non-protein components (e.g. nucleic acids) with the same absorption spectrum since it may lead to erroneous results. Aside from determining protein concentrations, absorbance values can also be used in detecting conformational changes and ligand binding and for following enzyme reactions.
The Effect of Tryptophan and Tyrosine in Protein Quantitation
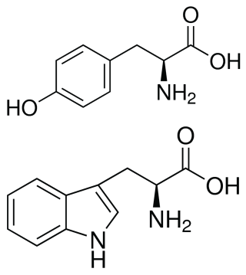
Due to the presence of tyrosine and tryptophan, proteins and peptides containing these aromatic amino acids absorb UV light at a wavelength of 280 nm. Each of these residues has distinct absorption and emission wavelengths and varies in quantum yields. Phenylalanine and disulfide bonds also contribute in the absorption at this wavelength but since it is relatively insignificant, it can only be observed in the absence of both tryptophan and tyrosine.
Many enzymatic cofactors containing aromatic ring structures (e.g. FMN, FAD, NAD and porphyrins) also absorb UV light for excitation and therefore add to the intensity of the resulting fluorescence. Special proteins such as Green Fluorescent Protein also have an internal serinetyrosine-glycine sequence that is modified post-translationally and is fluorescent in the visible light region.
Tryptophan
Tryptophan is significantly more fluorescent than tyrosine and phenylalanine. However, its fluorescent properties are solvent dependent, i.e. the spectrum shifts to shorter wavelengths and increases in intensity as the polarity of the solvent decreases. As such, tryptophan residues buried in hydrophobic domains of folded proteins exhibit a spectral shift of 10 to 20 nm.
Due to its greater absorptivity, higher quantum yield, and resonance energy transfer, the fluorescence spectrum of a protein containing the three amino acids usually resembles that of tryptophan.
Tyrosine
Tyrosine can be excited at wavelengths similar to that of tryptophan but will emit at a distinctly different wavelength. While it may be true that tyrosine is less fluorescent than tryptophan, it can provide significant signal since it is often present in large numbers in many proteins. Tyrosine fluorescence has been observed to be quenched by the presence of nearby tryptophan moieties either through resonance energy transfer or the ionization of its aromatic hydroxyl group.
Here are some important points to remember when measuring peptides using the A280 method.
- Proteins of similar molecular weight can have different absorbance values due to the difference in tryptophan and tyrosine content.
- UV absorbance is also affected by protein structure. Thus, conditions which affect structure (such as temperature, pH, ionic strength, or the presence of detergents) can affect the ability of aromatic residues to absorb light at 280 nm, and change the value of the protein’s extinction coefficient.
- The local environment of the aromatic amino acids can have an effect on their spectra. This means that tryptophan will have an emission peak at lower wavelengths when buried within the hydrophobic inner regions of a protein while tyrosine will often transfer its energy to adjacent tryptophan amino acids. Ionized tyrosinate, which forms when protons are lost from tyrosine as a result of increasing pH, will demonstrate wavelengths similar to tryptophan.






