 DNA denaturation is the process of breaking down the DNA molecule, generally for the purposes of comparison or sequencing. As with many laboratory techniques, there are a variety of ways to denature DNA -- and each of them tend to be better for specific applications. The top three methods of DNA denaturation are heat, NaOH treatment, and salt. Each of these methods will break the bonds between strands, but may do so with a greater degree of accuracy or lessened disruption.
DNA denaturation is the process of breaking down the DNA molecule, generally for the purposes of comparison or sequencing. As with many laboratory techniques, there are a variety of ways to denature DNA -- and each of them tend to be better for specific applications. The top three methods of DNA denaturation are heat, NaOH treatment, and salt. Each of these methods will break the bonds between strands, but may do so with a greater degree of accuracy or lessened disruption.
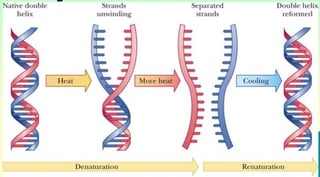
DNA Denaturation through Heat
DNA can be denatured through heat in a process that is very similar to melting. Heat is applied until the DNA has unwound itself and separated into two single strands. Once the strands have been separated, the DNA will then be cooled back down to a stable temperature. The process of cooling allows molecules to be formed in the DNA mixture, which then produces certain sequences that can be looked at as markers. Heat denaturation is frequently used when comparing the differences between species. Though DNA melting is a fairly simple and straightforward process, it is generally not used when accuracy is required. Heated DNA denaturation is considered to be less accurate than DNA sequencing and is used for more broad scope applications. This type of denaturation may also be used within the polymerase chain reaction.
DNA Denaturation through NaOH Treatment
Apart from heating, chemical denaturation can also be achieved through the use of NaOH. A certain concentration of NaOH can be used to denature DNA safely, often in as little as one minute. As the concentration of NaOH used is reduced, the process will take longer -- but the DNA can still be fully denatured. NaOH has been shown to be one of the most effective and reliable methods of complete denaturing. Other chemicals, such as formamide, are not able to denature DNA as rapidly or as reliably. Because NaOH can be used at a variety of concentrations, it is also able to be scaled quite easily. Furthermore, the DNA that is denatured with NaOH can be renatured through the use of a phosphate buffer. DNA that is denatured through other chemicals, such as DMSO, are not able to be fully renatured in this fashion -- and this can lend NaOH to more applications.
DNA Denaturation through Salt
A high concentration of salt will cause DNA to naturally denature, given the right concentration of salt. DNA denaturation with salts are similar to denaturation through the use of organic solvents. In general, DNA denaturation through salt cannot be renatured. Salt is often used in addition to an acid for the full denaturation of DNA, and it may also be used in conjunction with heat. Salt is not usually used as the sole process of denaturation -- it's usually used alongside other chemicals such as isopropanol and ethanol. This process is able to be used on larger volumes of DNA, which makes it less useful for highly accurate and specific work, but more useful for scaling up and processing DNA in larger quantities.
Though there are many techniques associated with DNA denaturation, the end result is the same: the bonds between the strands are broken and new molecules are formed, which can then be compared as desired. The ideal process of DNA denaturation depends on what the DNA needs to be used for, how accurate and specific the comparisons need to be, and the volume of material that has to be processed. In general, both heat and salt denaturation can be easily scaled and used with larger quantities, while NaOH denaturation may be slightly more accurate and useful in smaller quantities.






