Recombinant proteins are usually expressed with an affinity tag that facilitates binding of the protein to an affinity matrix and results in high yield purification of proteins (usually above 90% yield) by exploiting avidity between the tag and affinity matrix. Tags are usually small peptides or proteins that facilitate selective binding to an affinity matrix. There are many types of tags available to facilitate protein purification, some common examples are poly-histidine tag, GST tag, FLAG tag. The bound proteins can be eluted using a chemical agent usually known as eluent that has a higher affinity for affinity matrix and results in the release of bound tagged proteins from the affinity matrix by chemically competing against the bound proteins for the binding sites on affinity matrix.
Once the purification of proteins is complete these tags may become unwanted because they interfere with the biophysical and biochemical attributes of the purified protein. Tags can (a) change protein stability (b) protein conformation (c) inhibition of enzymatic activity (d) alterations in biological function/activity (d) undesired changes in protein structure for structural studies (e) toxicity. For example, one of the most commonly used tag, ‘hexahistidine tag’ can influence protein folding, stability and thermotolerance. Not all tags behave similarly, some affinity tags such as MBP and GST tags can significantly improve protein solubility and stability.
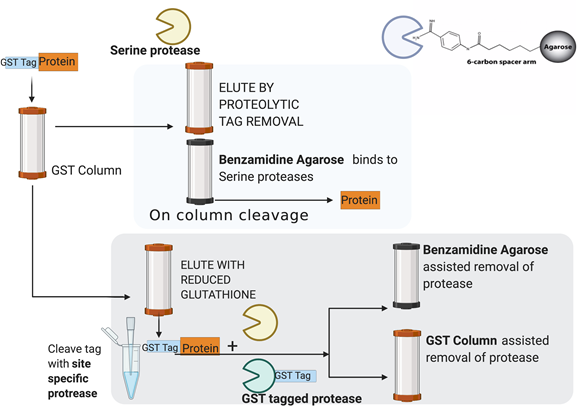
Figure legend: Schematic of On column and off column tag removal of tagged recombinant proteins, on column tag removal means the cleavage of tag while recombinant tagged protein is bound to the affinity matrix. It requires a subsequent capture of serine proteases by benzamidine agarose column which is used in tandem with the affinity matrix column. In off column mode, the tag is cleaved off the eluted protein with a serine protease and the cocktail is then passed through either a Benzamidine agarose column or a suitable affinity column which can capture the tagged protease.
Since tags can have desirable as well as non-desirable effects on protein structure and behaviour and it is difficult to predict the effect of tag on protein and its behaviour, it is usually desirable to remove the tag once the purification of protein has been done. This becomes even more relevant when the recombinant protein is to be used for therapeutic purposes.
Usually, there is a linker sequence between the protein and the tag, the linker has a site for the action of proteolytic enzymes. The proteases can digest the sequence and remove the tag. The tag can be removed by the action of proteases such as enterokinase, thrombin and factor-Xa. G- Biosciences recommends the use of highly effective recombinant enterokinase that can be used to remove the FLAG tag.
On column Tag removal
Removal of a tag from proteins may require large quantities of proteases owing to high ratios of the enzyme to protein required for optimal activity. Therefore, on column tag removal is considered more effective for large scale purifications because it allows for better protein recovery and also reuses protease and columns. As shown in a self-explanatory figure above, the tag is removed by the action of a serine protease and protein is eluted from the affinity matrix along with the protease. Since protease co-elutes with protein, it needs to be removed.
Sometimes the protease used is tagged with a suitable affinity handle such as biotin or GST to enable its removal from the eluted material but when it is not tagged, Benzamidine Agarose is used. Benzamidine binds to proteases with a high affinity. G biosciences offer p-aminobenzamidine agarose where the benzamidine group is linked to agarose beads through a long 6 carbon spacer arm. The 6- carbon spacer arm between the ρ-Aminobenzamidine group and the agarose beads makes it suitable for coupling of small proteins and peptides. The long spacer arm minimizes steric hindrance allowing high efficient binding of ligands, including small proteins and peptides.
Off column Tag removal
The eluted protein is treated with protease to remove the tag in a reaction mixture, this requires additional steps to purify the recombinant proteins from protease impurity. Usually, for an off column application, it is preferred to use a tagged protease. The removal of tag from protein can be done by passing the eluent through a benzamidine column or an appropriate affinity matrix to enable removal of tagged protease. Proteases can be tagged with biotin, GST tag or even His tags to facilitate their removal.