 As a common technique in molecular and cell biology, Western blot analysis allows you to detect specific proteins in a tissue homogenate or extract. However, if you want to ensure the success of your Western blot experiment, you need to learn how to properly prepare samples.
As a common technique in molecular and cell biology, Western blot analysis allows you to detect specific proteins in a tissue homogenate or extract. However, if you want to ensure the success of your Western blot experiment, you need to learn how to properly prepare samples.
What is Western Blot Analysis?
Commonly used in research and diagnostic laboratories, Western blot analysis is an important technique that detects specific proteins in a sample of tissue homogenate or extracts and separates proteins based on their molecular weight.
Proteins are first separated by polyacrylamide gel electrophoresis (PAGE). Next an electrical current is passed through the polyacrylamide gel and the proteins are transferred from the polyacrylamide gel onto the membrane, which creates an exact copy of the protein separation pattern observed in the original gel.
After transferring the sample to the membrane, the next step is blocking the membrane. If you fail to do so, the antibodies that bind to nonspecific binding sites on the membrane will make it difficult to detect the presence of the target protein, leading to false positives.
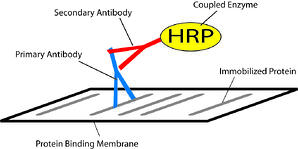
Once you immobilize the proteins on a protein binding membrane, you can probe them with a primary antibody, which is specific to the protein of interest. After you bound it, you can visualize the antibody with either with a particular tag coupled to the primary antibody or with a second antibody.
How to Prepare Your Samples
When preparing samples for Western blot analysis, there are four specific steps you must follow:
1. Choose the Right Lysis Buffer for Your Sample
To ensure the success of your experiment, you must prepare and choose the correct lysis buffer for your sample. If you use an improper lysis buffer, you will have a difficult time extracting the proteins of interest.
For a successful Western blot test, you need to isolate proteins from cells and tissues via lysis, which breaks down the cell membrane to separate proteins from the non-soluble parts of the cell.
Choosing the proper lysis buffer depends on the sub-localization of the protein. The process of selecting a lysis buffer and determining an appropriate volume is usually a process of trial and error. Lysis buffers vary from gentle ones with no detergent, to harsher, denaturing buffers with multiple detergents.
2. Add Protease and Phosphatase Inhibitors
During cell lysis, you need to add protease inhibitors and possibly phosphatase inhibitors to protect the proteins from enzymes released during sample preparation.
Protease and phosphatase inhibitors prevent fast proteolysis and dephosphorylation of the targets upon cell lysis. To slow protein degradation, you must prepare the buffers before every experiment, and keep cells and buffers on ice or at 4 ̊C during the entire procedure.
3. Determine Protein Concentration
To ensure equal loading of the SDS-PAGE gel, you must determine the protein concentration of every lysate and normalize it across the samples of every experiment. With equal loading, you can accurately quantify protein levels and expression differences in Western blotting.
To determine protein concentration, you can use a variety of protein assay methods, including the Lowry assay, the Bradford assay, the BCA assay, and UV spectroscopy. At G-Bio, we advise loading approximately 15-30 μg of the protein lysate to ensure a proper range of protein detection.
4. Load the Proteins
After determining protein concentration, you must dilute the protein samples in the gel loading buffer, otherwise known as 2x Laemmli sample buffer. The buffer contains reducing and denaturing agents, including SDS, mercaptoethanol, and glycerol.
Glycerol helps samples sink easily into the wells of the gel. The buffer also contains bromophenol blue, a tracking dye that reaches the bottom of the gel that indicates the end of electrophoresis.
Before loading the samples, you must heat them for 5 minutes at 100°C, or 10 minutes at 70°C to aid in the denaturation. Once the samples are heated, they can be loaded into the gels immediately, or placed at 4°C or -20°C for later analysis.
Choose G-Biosciences for Your Western Blot Analysis Needs
At G-Biosciences, we offer a variety of products to suit your western blot experiments. To learn more about our products and place an order, visit our website today!






