Culturing cells in the dishes has been the most critical and an important tool in the field of drug discovery and development for decades. Cell culture based assays allow a simple yet cost-effective technique bypassing tedious and tiresome animal-testing. This also provides a controlled situation, which can provide results based on the responses of chosen cells due to any stimuli or drug. For the past few decades, many of the methods were developed keeping the conventional two-dimensional monolayer culture in consideration where a single layer of cells is grown on a flat surface having a matrix on it. Nonetheless, 2D culture came as a boon for cell-based assays, but it did have its own share of limitations. The most important of all is the inadequate consideration of in-vivo environment where the cells are surrounded by the extracellular membrane in a 3-dimensional manner while 2D cultures do not consider cell-cell interaction in a 3D fashion providing an incoherent data.
These limitations were counteracted by the development of new culture system where cells were grown in a group or in a spheroid system, known as 3-dimensional or organoid culture. Since, the cells are grown in a close vicinity to each other; hence the behaviour of these cultured cells is closed to mimicking in-vivo condition reflecting the microenvironment residing in the tissues.
From the inception of this method till today, it has become a remarkable tool in cancer biology, regenerative medicine, stem cell biology, tissue implantation etc.
What is a 3D culture?
In 3-dimensional culture, the cells are grown in the form of aggregates or spheroids held together with the help of a matrix or a scaffold or in a scaffold free condition. The spheroids can be created by seeding cell suspension on a matrix (biological/synthetic) or polymerise the cell clumps in a quasi-liquid matrix. Most commonly used synthetic scaffolds are Polyethylene Glycol (PEG), polycaprolactone (PLA), polyvinyl alcohol (PVA) and polylactide-co-glycolide (PLG). Biologically derived scaffolds are commercially available as Matrigel, Basement membrane extract and hyaluronic acid.
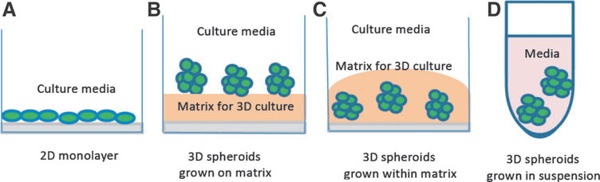
Fig.1. Schematic diagrams of different culture systems. Source: Edmondson et al, Assay and drug development technologies, 2014.
Factors to be considered for 3D cultures:
- Cell source: A variety of cells can be used to generate 3D cultures. It can be obtained from progenitor cells, multipotent cells, stem cells etc. Also genetically modified variants of these cell populations, different cell lines or primary cells can be utilised for generating spheroids. Recent studies have open new avenues with induced pluripotent stem cells (iPSCs), thereby allowing use of different tissues for 3D cultures.
- Matrices or Substrates: Based on the matrix, two types of 3D cultures are developed: 3D culture with scaffold or without scaffold.
- Scaffold-based 3D cultures: Scaffolds are used in 3D to allow support and strengthening to the spheroid. It allows binding of the cells in a 3-dimensional manner. Since the scaffold is porous, it easily permits movement of oxygen, nutrients and excretion of waste products. Add on to these benefits, the cells can easily proliferate and migrate. Scaffolding allows growth of bigger spheroids, hence play a crucial role in drug screening, pre-clinical studies and tissue engineering.
Before proceeding to 3D cultures, it should be noted that the scaffold material should be compatible with the tissue of interest allowing all the vital functions of the cells. The scaffolds can be synthetic, generally used for in-vitro 3D cultures or biomedically-engineered scaffolds used for tissue regeneration. The scaffolds can be made into different shapes as membranous (sheets), quasi-liquid (hydrogels) and matrix form. Materials derived from metals, glasses or ceramics can be utilised for making scaffolds. - Scaffold-free 3D cultures: In this method, cell clumps are not grown on a solid support and the spheroids are smaller and fragile. Three different methods can be employed for scaffold-free 3D cultures: Forced-floating method, hanging drop method and agitation based method.
Forced-floating method harvests the use of low adhesion polymer-coated plates. The cell aggregates are laid on the plate after centrifugation of cell suspension.
Hanging drop method uses microwell inverted plates on which the cell suspension aliquots hang in the form of droplets allowing the cells to aggregate and create homogeneous 3D spheroids.
Agitation based methods use rotating bioreactors to create cell aggregates which don't stick to the reactor and keep moving gradually in the reactor.
- Scaffold-based 3D cultures: Scaffolds are used in 3D to allow support and strengthening to the spheroid. It allows binding of the cells in a 3-dimensional manner. Since the scaffold is porous, it easily permits movement of oxygen, nutrients and excretion of waste products. Add on to these benefits, the cells can easily proliferate and migrate. Scaffolding allows growth of bigger spheroids, hence play a crucial role in drug screening, pre-clinical studies and tissue engineering.
- Media support: Bath media or growth media is a cocktail of essential growth factors, nutrients and serum proteins. It is essential for the development, differentiation and growth of the cells. Requirement and composition of growth media varies according to the cell type to be grown. The growing conditions, nutrient requirements, serum or serum-free condition has to be optimized according to the cells and experimental requirement. The whole purpose of media support is to provide an isotonic environment with optimal pH. The media support has to be changed at regular intervals in order to replenish depleting nutrients and to avoid accumulation of toxic excretory molecules.
Comparison of 3D culture vs. 2D culture:
|
Cellular Properties |
2-Dimensional culture |
3-Dimensional culture |
|
Cellular morphology |
Monolayer (flattened cells) |
Aggregated as small organoids or spheroids |
|
Proliferation kinetics |
Faster than in-vivo |
Faster/Slower than 2D cultures depending on the cell types |
|
Growth media/ Drug exposure |
Even |
Uneven due to the compact structure |
|
Cell growth phase |
Mostly all cells in one phase |
Multiphasic cells (dividing/quiescent/ necrotic) |
|
Translatome/Genome expression |
Differential expression, not very close to in-vivo system |
Closer to in-vivo expression |
|
Drug response |
Highly and evenly responsive |
Variable response, mimic in-vivo response |
|
Environmental control |
Highly controlled uniform conditions |
Heterogeneous conditions |
|
Growth factor distribution |
Rapid |
Slow and uneven, leads to necrosis in the core cells |






