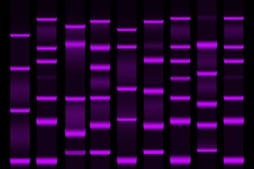
 When proteins need to be segregated out from a sample, protein electrophoresis may be used. Protein electrophoresis utilizes a matrix and an electrical current in order to easily separate proteins without disruption. Protein electrophoresis is beneficial for a few reasons: it can be performed on a relatively small sample size, it is accurate, and it is simple. There are a few different methods of protein electrophoresis, which may or may not involve a denaturing gel.
When proteins need to be segregated out from a sample, protein electrophoresis may be used. Protein electrophoresis utilizes a matrix and an electrical current in order to easily separate proteins without disruption. Protein electrophoresis is beneficial for a few reasons: it can be performed on a relatively small sample size, it is accurate, and it is simple. There are a few different methods of protein electrophoresis, which may or may not involve a denaturing gel.
The Process of Protein Electrophoresis
There are a few different techniques that may be used throughout protein electrophoresis depending on the nature of the sample and the needs of the laboratory. Native gel methods use non-denaturing gels, which thus analyzes the proteins while they are still folded. There are limitations to native gel methods -- because the proteins are still folded, the actual physical attributes of the protein may have an impact on the readability of the results. Native gel protein electrophoresis may be done as a blue native PAGE, clear native PAGE, or quantitative native PAGE.
Denaturing gel methods are considered to be a little more accurate. In denaturing gel methods, proteins are separated while they are unfolded. This will produce an easier to read result. SDS-PAGE is the most commonly used method, which involves a detergent agent known as sodium dodecyl sulfate. This SDS mixture is able to treat proteins, creating a uniform charge density. The SDS will form a bond with the proteins, thereby ensuring that they conform to a rod-like pattern.
Regardless of the gel, once the proteins have been introduced into the gel and buffer system, an electric charge is applied. The proteins will then move throughout the solution based on their electrophoretic mobility. This will separate out the denatured proteins. Once the proteins have been separated out, they are often stained using a protein stain. Like the other products used throughout protein electrophoresis, the type of staining may be determined by the type of protein and gel. The most popular type of protein stain is Coomassie Brilliant Blue. Coomassie Brilliant Blue binds to proteins and the excess dye can be removed through the process of de-staining -- but it is not the most sensitive method used. Silver staining can also be used when it is necessary to be stain trace amounts of proteins.
Buffer Systems for Protein Electrophoresis
The buffer system utilized throughout the process of electrophoresis is important to the outcomes. Buffer systems will ensure that the resulting bands are easier to read. Many protein electrophoresis processes are completed in a discontinuous buffer system. This system makes it easier to distinguish between individual bands by stacking the proteins on top of another, thereby making it easier to read the amounts of the individual proteins.
Buffer systems may have different areas of the gel with larger pores, thereby making it easier for certain types of protein to move through -- and preventing the migration of proteins that are larger still. Resolving gels have smaller pore sizes, which are able to successfully separate out different proteins based on their electrophoretic mobility. By stacking these within the buffer system, better results are able to be achieved.
Protein electrophoresis is a very useful way to analyze the protein distribution of any small sample. Because it relies heavily on gels, buffers, and staining, a laboratory will need to ensure that they have the right chemicals for the processes first. In general, protein electrophoresis is one of the easiest ways to analyze a fluid or extract for its contents -- and there are many different types of techniques that can be utilized depending on the fluid and the accuracy that is needed.





