 Gel filtration is a method of separating molecules by size. Among similar techniques, gel filtration is known as being a very simple and gentle procedure. When it comes to desalting and buffer exchange, gel filtration is often the preferred method as it can achieve better separation without any complex processes. Gel filtration is commonly used for chromatography, with the only negative being that it is very difficult to get high resolution results.
Gel filtration is a method of separating molecules by size. Among similar techniques, gel filtration is known as being a very simple and gentle procedure. When it comes to desalting and buffer exchange, gel filtration is often the preferred method as it can achieve better separation without any complex processes. Gel filtration is commonly used for chromatography, with the only negative being that it is very difficult to get high resolution results.
The Process of Gel Filtration
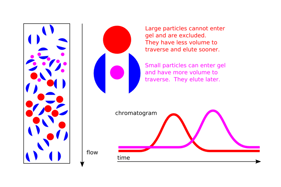
Gel filtration begins with a column, which is filled with both gel medium and beads. The beads involved are a special substance that will not absorb or react to any of the chemicals present. Smaller molecules will be able to enter these beads, but larger molecules will not. Thus, the smaller molecules will be inside and between the beads while the larger molecules will be located within the gel itself. The beads are generally made out of substances such as agarose, dextran, or polyacrylamide. Once the gel filtration has been completed, it will commonly be used for gel filtration chromatography.
Gel filtration is generally preferred over processes such as dialysis due to its speed. The process of dialysis can take hours whereas the process of gel filtration may take only a few minutes. Gel filtration can also be used on relatively small amounts of substances compared to dialysis, which makes it particularly useful for substances such as toxic or radioactive materials.
Desalting and Buffer Exchange
Removing salt from a sample is called desalting. Desalting may also include removing unincorporated nucleotides or phenol, or separating excess crosslinking. A buffer exchange occurs when salts are removed from a sample and replaced with a different type of salt. In general, gel filtration chromatography is used for these processes. In a desalting, the chromatography column is equilibrated with water. In buffer exchange, the chromatography column is equilibrated with the final type of buffer salts. Either way, the salts within the solution will be replaced, either by water or a different buffer salt. With gel filtration, buffer salts and other small molecules within the substance will slide through the resin beads. Faster molecules within the solution will be separated from the smaller and slower molecules, thereby separating the larger molecules out.
In a buffer exchange, the original buffer salts will be retained within the resin while the rest of the solution can then be removed. Buffer exchanges are frequently used to exchange one type of salt for a salt type that is more suitable to the current applications. This can occur before affinity chromatography, ion exchange, or electrophoresis
When dealing with desalting and buffer exchange, the column size of the gel filtration will be extremely important. Columns that are too large will lead to the dilution of the protein sample whereas columns that are too small could potentially lead to insufficient separation. Gravity-flow columns, chromatography cartridges, and centrifuge columns may all be used throughout this process. These columns represent differences in how the sample is moved through the filter matrix. Gravity-flow columns simply rely upon gravity to bring the molecules to rest within the resin. Cartridges are closed systems with forced liquid and centrifuge systems spin in order to force the samples through. In general, gravity-flow samples will take the longest while centrifuge systems will be much faster. Centrifuge systems may also lead to a less diluted sample.
Gel filtration is a fast, simple method of desalting a solution or performing a buffer exchange. With a gel medium and resin bed, larger molecules can easily be removed from smaller molecules, within a matter of minutes rather than hours. More importantly, this occurs without significant disruption or dilution to the sample itself.






