Question:
What are the common techniques used in protein fractionation?
The Protein Man Says:
 Protein fractionation generally refers to the process of isolating, identifying and characterizing various proteins present in a sample. However, the analysis of proteomes is usually hindered by the vast amounts of proteins, especially since the larger, more abundant proteins tend to inhibit the signal of lower abundance proteins. Incidentally, the lower abundance proteins are usually the more interesting proteins in the group.
Protein fractionation generally refers to the process of isolating, identifying and characterizing various proteins present in a sample. However, the analysis of proteomes is usually hindered by the vast amounts of proteins, especially since the larger, more abundant proteins tend to inhibit the signal of lower abundance proteins. Incidentally, the lower abundance proteins are usually the more interesting proteins in the group.
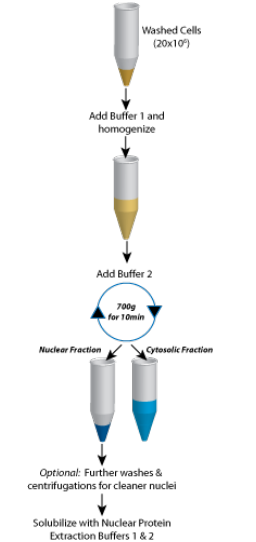
To address this problem, you need to identify the properties that separate them from the rest of the unwanted proteins and then use the appropriate protein fractionation technique(s) to isolate them from the rest of the unwanted proteins. You can use various general properties such as size, shape, solubility, stability and sedimentation velocity, ability to bind with various ionic groups, and affinity for substrates as a basis for isolating your protein of interest from complex biological samples. You can likewise separate proteins based on their cellular location, thereby allowing you to extract cytoplasmic, nuclear and membrane proteins.
Fractionation of proteins in solutions can usually be carried out through precipitation and/or chromatographic and electrophoretic procedures. Fractionation through precipitation can be achieved by "salting out" with ammonium sulfate, isoelectric precipitation (adjusting the pH to precipitate the unwanted proteins), or by using solvents such as alcohol or acetone.
By "salting out" or increasing the ionic strength of the solution, proteins that have more hydrophobic regions will aggregate and precipitate faster than those with less hydrophobic regions. Adjusting the pH, on the other hand, cause some of the proteins to reach their isoelectric point. At this point, some of the proteins begin to precipitate. It is interesting to note that this technique is mostly used to precipitate the unwanted proteins, leaving the protein of interest in the solution.
Proteins can also be fractionated by using chromatographic and electrophoretic procedures. Different proteins can be separated by employing gel filtration which separates proteins based on their size and shape and/or adsorption chromatography.
Keep in mind that there is no single way by which you can separate proteins so you need to establish what your final goals are before using a particular protein fractionation method. In addition, since there would rarely be a single fractionation technique that is suitable for most cases, you may require multiple, systematic techniques to complete your research.






